Abstract
Subunits of a proline-rich coccidioidal antigen (Ag2/PRA) of Coccidioides immitis were analyzed by comparison as vaccines in mice. The optimal dose of plasmid vaccine encoding full-length Ag2/PRA was determined to be between 10 and 100 μg. Mice vaccinated with plasmids encoding amino acids (aa) 1 to 106 were as protective as full-length Ag2/PRA (aa 1 to 194). The subunit from aa 27 to 106 was significantly but less protective. Plasmids encoding aa 90 to 151 or aa 90 to 194 were not protective. Analogous results were obtained with recombinant vaccines of the same amino acid sequences. In addition, mixtures of aa 90 to 194 with either aa 1 to 106 or aa 27 to 106 did not enhance protection compared to the active single-recombinant subunits alone. Humoral response of total immunoglobulin G (IgG) and subclasses IgG1 and IgG2a were detectable in subunit vaccinations but at significantly (100-fold) lower concentrations than after vaccination with plasmids encoding full-length Ag2/PRA. Since virtually all protection by vaccination with full-length Ag2/PRA can be accounted for in the first half of the protein (aa 1 to 106), this subunit could make a multicomponent vaccine more feasible by reducing the quantity of protein per dose and the possibility of an untoward reactions to a foreign protein.
Coccidioidomycosis is the consequence of infection with the fungus Coccidioides immitis (20). A few infections require extensive or lifelong antifungal treatment (8), especially if exposure is more intense (3, 5, 7, 19, 21, 26). To prevent some or all of the complications of coccidioidomycosis by vaccination would seem feasible, since most infections resolve spontaneously (11) and engender a high level of resistance to reinfection. Whole-cell vaccines protected mice if relatively large doses of vaccine were used (13, 14). In a human trial of the whole-cell vaccine, there was substantial local inflammation at the injection site, making it unacceptable (16, 22). Further attempts to develop a coccidioidal vaccine for humans have focused on subcellular and recombinant preparations (15).
The proline-rich coccidioidal antigen Ag2/PRA is a 194-amino-acid (-aa) protein which is a component of a glycopeptide. In previous studies, either protein vaccines using recombinant Ag2/PRA (rAg2/PRA) or DNA vaccines based on the sequence encoding Ag2/PRA demonstrated protection from otherwise lethal coccidioidal infection in mice (1, 9, 12, 19a). However, these studies also indicated that protection from Ag2/PRA in murine models of coccidioidal infection is not as complete as that obtained with killed whole-cell vaccines. Although it is possible that a vaccine candidate prepared with rAg2/PRA as the only antigenic component would be sufficiently effective to be of practical benefit, it is also possible that a more useful vaccine might be obtained by immunization with two or more coccidioidal antigens. If more than one component is to be used, reducing each protein to the essential domain responsible for protection may be important to minimize the size of the vaccine dose and to reduce possible untoward side effects of immunization.
Zhu et al. used recombinant truncations of Ag2/PRA to detect antibody binding of human immune serum to aa 19 to 79 but not to aa 19 to 61, aa 49 to 79, or aa 62 to 194 (25). To extend this work, we used a computer algorithm to identify putative antigenic domains and also analyzed the sequence for common structural motifs as has been done by others (23). In this report, we used both DNA and protein vaccines prepared with subunits of Ag2/PRA to further define the antigenic domain responsible for protection.
MATERIALS AND METHODS
Mice.
Female, 6-week-old BALB/c mice were purchased from Harlan-Sprague-Dawley (Indianapolis, Ind.).
Design of Ag2/PRA subunits.
We used PEPTIDESTRUCTURE (GCG Package; Genetics Computer Group, Madison, Wis.) as a guide in designing four overlapping subunits of Ag2/PRA. In order not to miss potential epitopes, we divided the full-length protein approximately in half with a 17-aa overlap (aa 1 to 106 and aa 90 to 194). For additional studies, we also prepared internal subunits corresponding to aa 27 to 106 and aa 90 to 151 to encompass and overlap specific subunits suggested by structural analysis.
Construction of plasmid vaccines.
A mammalian expression vector, VR1020 (Vical, Inc., San Diego, Calif.), was used to construct the DNA vaccine, pCVP20.17, encoding the full-length sequence (1) and subunit sequences of Ag2/PRA (primer sequences and PCR conditions available on request). The orientation, frame, and sequence of plasmid inserts were confirmed by DNA sequencing.
For selected studies, a plasmid encoding murine interleukin-12 (IL-12) (pVR4001; Vical) was also used. In preliminary studies we confirmed by our immunization procedures that the plasmid encoding IL-12 by itself had no protective effect against a coccidioidal infection.
Expression of recombinant peptides.
Procedures for expression and purification of rAg2/PRA have been previously described (12) and followed for the subunits. Optimal expression was as follows: for the subunit encoding aa 1 to 106 (subunit 1-106), pET32a vector in BL21(DE3) cells grown in terrific broth; for subunit 27-106, pET28a vector in BL21(DE3)star cells grown in Luria broth; and for subunit 90-194, pET28a vector in Tuner(DE3)plysS cells grown in terrific broth. After expression, each recombinant peptide was separated from its fusion partner, concentrated, and stored at −70°C until use. Purity of recombinant proteins was greater than 95% as determined by Coomassie blue staining of electrophoretic separations (18) using 16.5% Tricine acrylamide gel (Bio-Rad, Hercules, Calif.).
Protection studies.
Immunizations were carried out as previously described with groups of eight or more mice (1). Mice were vaccinated twice 4 weeks apart. One month after the second injection, mice were infected intraperitoneally (51 ± 13 arthroconidia per mouse [mean ± standard error of the mean]) with strain rs of C. immitis as previously described (1). Fourteen days after infection, all mice were sacrificed with an overdose of inhalant anesthesia, and the spleen and right lung were removed aseptically from each mouse. CFU were enumerated at 3 days and reported as log10 CFU per organ.
Serologic assays.
Mice were vaccinated as above. Four to five weeks after booster immunization, uninfected mice were anesthetized and exsanguinated via the retro-orbital plexus; blood was collected using a heparinized, capillary-based collection system (Microvette CB300; Sarstedt, Newton, N.C.) and centrifuged, and the plasma was harvested and frozen at −20°C until titration for total IgG, IgG1, and IgG2a as previously described (1). Briefly, plasma was diluted in fourfold serial dilutions and applied in triplicate to microtiter plates coated with full-length rAg2/PRA. Antibody was detected with a horseradish peroxidase-conjugated anti-immunoglobulin, and absorbance was measured by an optical density reader. The titer was determined as the highest dilution that was twice the level of the background.
Statistical analyses.
For fungal burden of organs of infected animals, comparisons between groups were determined by analysis of variance and Duncan's multiple-range tests as implemented by SAS software (version 6.12; SAS Institute, Inc., Cary, N.C.). The type I experiment-wise error rate of 0.05 was used to control for multiple comparisons.
Comparisons between immunoglobulin titers of mice immunized with different plasmid vaccines utilized the Kruskal-Wallis test (Systat; SPSS, Inc., Chicago, Ill.).
RESULTS
Protection against infection with C. immitis in relation to Ag2/PRA DNA vaccine dose.
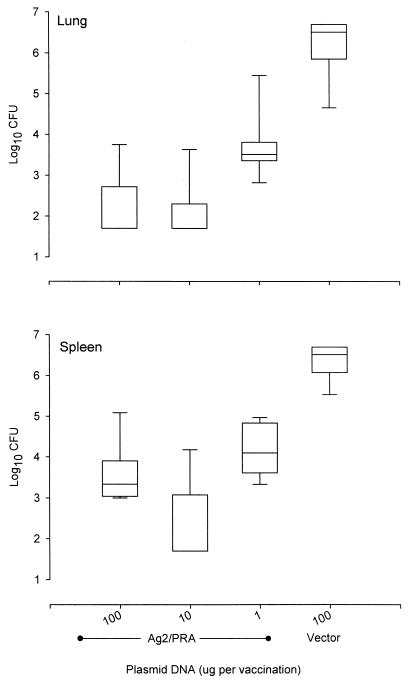
In a previous study, vaccination with 100 μg of plasmid containing cDNA encoding full-length Ag2/PRA (pCVP20.17) conferred protection on BALB/c mice infected intraperitoneally with C. immitis (1). In the present study, lower doses of pCVP20.17 were assessed (Fig. 1). Compared to the vector alone, all vaccine doses produced significant reductions in colonies in both lungs and spleens (P < 0.05). In cultures of lungs, both the 100- and 10-μg doses produced significantly greater reductions in colonies than did the 1-μg vaccine dose (P < 0.05). In cultures of spleens, the 10-μg dose was significantly more protective than either 1- or 100-μg doses (P < 0.05). Based upon these results, 20 μg of plasmid was used in subsequent studies.
FIG. 1.
Comparison of protection afforded by different vaccine doses of plasmids encoding full-length Ag2/PRA. Each box represents the range for 25% of the results above and below the group median (indicated by the horizontal line within the box). The vertical lines indicate the 95% confidence range for the group.
Differential effects of DNA subunit vaccines.
As shown in Fig. 2, significantly fewer colonies of C. immitis were isolated from lungs of mice vaccinated with plasmids encoding either aa 1 to 106 or aa 27 to 106 than from mice receiving the vector alone (P < 0.05). Although subunit 1-106 was similar to full-length antigen, subunit 27-106 was less effective (P < 0.05). In contrast, there was virtually no effect of vaccination with plasmids encoding either aa 90 to 151 or aa 90 to 194.
FIG. 2.
Comparison of protection afforded by vaccination with plasmids encoding either full-length Ag2/PRA or its different subunits. The results are from two separate studies (indicated by different hatchings) which yielded similar results. See Fig. 1 legend for details.
Although there appeared to be no protection from the plasmid encoding aa 90 to 194 when used alone as a vaccine, it is still possible that this portion of the protein might contribute an adjuvant-like effect. In order to determine if our methods would detect such an effect, should it exist, we coadministered a plasmid encoding aa 27 to 106 with one encoding murine IL-12, which in a previous report by Jiang et al. enhanced protection by the full-length Ag2/PRA (10). In our hands, vector alone resulted in a mean log10 CFU of 5.74, vaccination with plasmid encoding aa 27 to 106 reduced the log10 CFU to 3.85, and vaccination with plasmid encoding aa 27 to 106 in combination with plasmid encoding IL-12 reduced log10 CFU to 1.70. Each of these groups was significantly different from the other two (P < 0.05). Also, log10 CFU after vaccination with plasmids encoding full-length Ag2/PRA was 2.11, a difference from vector alone similar to that found in other studies. That we obtained the same effect of the plasmid encoding IL-12 in combination with the subunit antigen as did Jiang et al. with the full-length antigen is useful for the interpretation of studies with combinations of recombinant peptide subunits described in the following section.
Protection afforded by recombinant peptide subunit vaccines.
As previously seen with plasmid vaccination (Fig. 2), mice vaccinated with full-length Ag2/PRA or either recombinant peptides 1-106 or 27-06 were significantly protected (P < 0.05) compared to mice receiving adjuvant alone (Fig. 3). Also similar to the previous study, vaccination with recombinant peptide 1-106 was more protective than was recombinant peptide 27-106 (P < 0.05). In contrast, vaccination with the recombinant peptide 90-194 showed virtually no protection. Furthermore, coadministration of recombinant peptide 90-194 with either of the N-terminal subunit recombinant peptides did not enhance their protection.
FIG. 3.
Comparison of protection afforded by vaccination with recombinant proteins of full-length Ag2/PRA or its different subunits. See Fig. 1 legend for details.
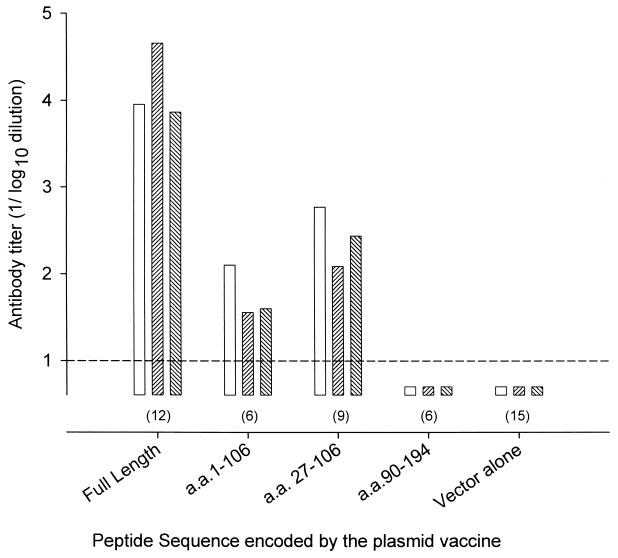
Humoral responses to subunit vaccination.
Mice vaccinated with plasmids encoding the full-length protein consistently developed higher titers of total IgG and both IgG subclasses compared to mice receiving plasmids encoding either aa 1 to 106 or aa 27 to 106 (Fig. 4) (P < 0.01 for all comparisons). Vaccination with vector alone or plasmids encoding aa 90 to 194 resulted in no detectable anti-Ag2/PRA antibodies. Moreover, mice immunized with mixtures of plasmids encoding aa 1 to 106 or aa 27 to 106 with aa 90 to 194 had humoral responses similar to those seen in mice immunized with plasmids encoding aa 1 to 106 or aa 27 to 106 alone (data not shown).
FIG. 4.
Humoral responses of mice vaccinated with plasmids encoding full-length Ag2/PRA or its subunits. Bars represent geometric mean titers for each group of mice. Open bars, total IgG; rising hatch, IgG1; falling hatch, IgG2. Numbers in parentheses indicate number of mice immunized for each group.
DISCUSSION
The studies reported here suggest that all of the vaccine protection afforded mice against infection with C. immitis exists within the first 106 aa of the 194-aa Ag2/PRA. In keeping with our previous report (1), protection from vaccination with subunits of Ag2/PRA was evident whether vaccination was carried out with plasmids encoding the antigen or with recombinant peptides in monophosphoryl lipid A (MPL) adjuvant. Some of this activity has been localized to the sequence from aa 27 to 106 (Fig. 2 and 3). However, this does not account for all of the protection that results from immunization with plasmids encoding full-length Ag2/PRA. In other studies not yet published and using a different murine model of coccidioidal infection than is described in the present report, we have found significant protection from the recombinant peptide 1-40 when administered with MPL adjuvant. Therefore, we believe that it is more likely that this sequence contributes specific protective epitopes to those present in aa 27 to 106.
In an effort to rationally design subunits for vaccination studies, we had analyzed the primary amino acid sequence of Ag2/PRA for functional domains, some of which are hallmarks of fungal cell wall proteins. As previously reported, aa 1 to 18 form a putative signal sequence for export to the cell surface via the endoplasmic reticulum (24). Between aa 26 and 83, there is an eight-cysteine motif of unknown function, recently described in proteins from two other pathogenic fungi (2, 6). The proline- and threonine-rich repeating sequences found between aa 98 and 141 are a common feature of fungal cell wall proteins, and in vivo, they may be highly O-glycosylated and cross-linked to cell wall polysaccharides. The C-terminal 23 aa form a putative glycosylphosphatidylinositol signal sequence. Absence of a two-lysine motif N terminal to the cleavage site suggests that Ag2/PRA is most likely not bound to the cell membrane via glycosylphosphatidylinositol (4) but rather is attached to the cell wall matrix. Thus, aa 1 to 18 and aa 172 to 194 are most likely not a part of the mature protein in vivo.
The antigen prediction algorithm that we used is based on surface properties of globular proteins and may have little to do with the processed peptides presented to major histocompatibility complex class I or class II. Algorithms which recognize class I epitopes are better developed (17), but these are restricted to specific inbred strains for a given species as these domains can be predicted only in the context of specific major histocompatibility complex. A search using one such database revealed no class I motifs for H-2d (BALB/c) in rAg2/PRA. Class II motifs are less well understood and may be more dependent on flanking regions that influence antigen processing than on internal peptide sequence (17). Although we used the functional and immunologic motifs as a framework on which to design the ends of our subunit peptides, we could not from this analysis have predicted that the protective activity of rAg2/PRA would be localized to the first 106 aa.
We have also addressed the possibility that aa 90 to 194 could provide adjuvant-like enhancement to the N-terminal half of Ag2/PRA. That plasmids encoding IL-12 enhanced the protection by plasmids encoding aa 27 to 106 (Fig. 3) provides evidence that such effects could be detected by our methods. However, we were unable to demonstrate a similar affect with recombinant peptide 90-194 mixed with either peptide 1-106 or 27-106. In these studies, all vaccines contained MPL as an adjuvant, so the possibility exists that aa 90 to 194 may have some adjuvant-like effect that was obscured by the concurrent use of MPL. However, we have previously demonstrated the need for an adjuvant to confer protection of full-length rAg2/PRA in BALB/c mice (1). Thus, whatever adjuvant-like effect the recombinant peptide 90-194 may have, it is likely to afford little benefit in excess of that provided by MPL or perhaps other generally available adjuvants.
Measurement of total IgG and its subclasses in sera from mice vaccinated with subunit vaccines demonstrated that both IgG1 and IgG2a were stimulated but that the antibody titers were approximately 100-fold lower than those produced with full-length antigen vaccine (Fig. 4). We presume that this difference is due to loss of tertiary structure that is present in the full-length antigen.
Based upon these studies, the subunit 1-106 could be substituted for full-length Ag2/PRA in formulations of practical vaccine candidates to prevent coccidioidomycosis. Such a selection would require less protein per dose, which may be of particular importance if Ag2/PRA is only one of several antigens. Moreover, the administration of any foreign protein as a vaccine carries with it a risk of either allergic or toxic untoward reactions. Therefore, to eliminate amino acid sequence that contributes nothing to protection should improve the safety profile of the vaccine candidate. Finally, one approach to production of multicomponent vaccines is to express them in sequence as a single-recombinant peptide. This potentially allows for simplification of the production process and decreased expense. Substituting a subunit for full-length Ag2/PRA would make this approach more feasible by reducing the overall size of the resulting multicomponent antigen.
Acknowledgments
This work was supported in part by the U.S. Department of Veterans Affairs, the California Health Care Foundation, and PHS research grant no. 5 P01 A/37232-06 from the National Institutes of Health.
The statistical assistance of Gretchen A. Cloud is gratefully acknowledged. We thank Theo Kirkland, University of California, San Diego, for providing arthroconidia.
Editor: T. R. Kozel
REFERENCES
- 1.Abuodeh, R. O., L. F. Shubitz, E. Siegel, S. Snyder, T. Peng, K. I. Orsborn, E. Brummer, D. A. Stevens, and J. N. Galgiani. 1999. Resistance to Coccidioides immitis in mice after immunization with recombinant protein or DNA vaccine of a proline-rich antigen. Infect. Immun. 67:2935-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun, B. R., W. S. Head, M. X. Wang, and A. D. Johnson. 2000. Identification and characterization of TUP1-regulated genes in Candida albicans. Genetics 156:31-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairns, L., D. Blythe, A. Kao, D. Pappagianis, L. Kaufman, J. Kobayashi, and R. Hajjeh. 2000. Outbreak of coccidioidomycosis in Washington State residents returning from Mexico. Clin. Infect. Dis. 30:61-64. [DOI] [PubMed] [Google Scholar]
- 4.Caro, L. H. P., H. Tettelin, J. H. Vossen, A. F. J. Ram, H. Van den Ende, and F. M. Klis. 1997. In silicio identification of glycosyl-phosphatidylinositol-anchored plasma-membrane and cell wall proteins of Saccharomyces cerevisiae. Yeast 13:1477-1489. [DOI] [PubMed] [Google Scholar]
- 5.CDC. 2001. Coccidioidomycosis in workers at an archeologic site: Dinosaur National Monument, Utah—July 2001. Morb. Mortal. Wkly. Rep. 50:1005-1008. [PubMed] [Google Scholar]
- 6.DeZwaan, T. M., A. M. Carroll, B. Walent, and J. A. Sweigard. 1999. Magnaporthe grisea Pth11p is a novel plasma membrane protein that mediates appressorium differentiation in response to inductive substrate. Plant Cell 11:2013-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon, D. M. 2001. Coccidioides immitis as a select agent of bioterrorism. J. Appl. Microbiol. 91:602-605. [DOI] [PubMed] [Google Scholar]
- 8.Galgiani, J. N., N. M. Ampel, A. Catanzaro, R. H. Johnson, D. A. Stevens, and P. L. Williams. 2000. Practice guidelines for the treatment of coccidioidomycosis. Clin. Infect. Dis. 30:658-661. [DOI] [PubMed] [Google Scholar]
- 9.Jiang, C., D. M. Magee, T. N. Quitugua, and R. A. Cox. 1999. Genetic vaccination against Coccidioides immitis: comparison of vaccine efficacy of recombinant antigen 2 and antigen 2 cDNA. Infect. Immun. 67:630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang, C. Y., D. M. Magee, and R. A. Cox. 1999. Coadministration of interleukin 12 expression vector with antigen 2 cDNA enhances induction of protective immunity against Coccidioides immitis. Infect. Immun. 67:5848-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerrick, S. S., L. L. Lundergan, and J. N. Galgiani. 1985. Coccidioidomycosis at a university health service. Am. Rev. Respir. Dis. 131:100-102. [DOI] [PubMed] [Google Scholar]
- 12.Kirkland, T. N., F. Finley, K. I. Orsborn, and J. N. Galgiani. 1998. Evaluation of the proline-rich antigen of Coccidioides immitis as a vaccine candidate in mice. Infect. Immun. 66:3519-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine, H. B., J. M. Cobb, and C. E. Smith. 1961. Immunogenicity of spherule-endospore vaccines of Coccidioides immitis for mice. J. Immunol. 87:218-227. [PubMed] [Google Scholar]
- 14.Levine, H. B., Y.-C. Kong, and C. E. Smith. 1965. Immunization of mice to Coccidioides immitis: dose, regimen and spherulation stage of killed spherule vaccines. J. Immunol. 94:132-142. [PubMed] [Google Scholar]
- 15.Pappagianis, D. 2001. Seeking a vaccine against Coccidioides immitis and serologic studies: expectations and realities. Fungal Genet. Biol. 32:1-9. [DOI] [PubMed] [Google Scholar]
- 16.Pappagianis, D., and Valley Fever Vaccine Study Group. 1993. Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans. Am. Rev. Respir. Dis. 148:656-660. [DOI] [PubMed] [Google Scholar]
- 17.Rammensee, H.-G., T. Friede, and S. Stevanovic. 1995. MHC ligands and peptide motifs: first listing. Immunogenetics 41:178-228. [DOI] [PubMed] [Google Scholar]
- 18.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Schneider, E., R. A. Hajjeh, R. A. Spiegel, R. W. Jibson, E. L. Harp, G. A. Marshall, R. A. Gunn, M. M. McNeil, R. W. Pinner, R. C. Baron, R. C. Burger, L. C. Hutwagner, C. Crump, L. Kaufman, S. E. Reef, G. M. Feldman, D. Pappagianis, and S. B. Werner. 1997. A coccidioidomycosis outbreak following the Northridge, Calif., earthquake. JAMA 277:904-908. [PubMed] [Google Scholar]
- 19a.Shubitz, L., T. Peng, R. Perrill, J. Simons, K. Osburn, and J. N. Galgiani. 2002. Protection of mice against Coccidioides immitis Intranasal infection by Vaccination with recombinant antigen 2/PRA. Infect. Immun. 70:3287-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens, D. A. 1995. Current concepts: coccidioidomycosis. N. Engl. J. Med. 332:1077-1082. [DOI] [PubMed] [Google Scholar]
- 21.Werner, S. B., D. Pappagianis, I. Heindl, and A. Mickel. 1972. An epidemic of coccidioidomycosis among archeology students in northern California. N. Engl. J. Med. 286:507-512. [DOI] [PubMed] [Google Scholar]
- 22.Williams, P. L., B. W. Brown, Jr., R. T. Cunningham, H. Einstein, R. Ellsworth, J. N. Galgiani, C. R. Hampson, C. W. Holeman, R. H. Johnson, T. R. Larwood, H. B. Levine, D. A. Massa, J. Nichols, D. Pappagianis, D. A. Stevens, and J. S. Tidball. 1985. Further assessment of the morbidity associated with the killed Coccidioides immitis spherule vaccine in humans, p. 339-346. In H. E. Einstein and A. Catanzaro (ed.), Coccidioidomycosis: proceedings of the 4th International Conference on Cocciidioidomycosis. National Foundation for Infectious Diseases, Washington, D.C.
- 23.Zhu, Y., C. Yang, D. M. Magee, and R. A. Cox. 1996. Coccidioides immitis antigen 2: analysis for gene and protein. Gene 181:121-125. [DOI] [PubMed] [Google Scholar]
- 24.Zhu, Y., C. Yang, D. M. Magee, and R. A. Cox. 1996. Molecular cloning and characterization of Coccidioides immitis antigen 2 cDNA. Infect. Immun. 64:2695-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu, Y. F., V. Tryon, D. M. Magee, and R. A. Cox. 1997. Identification of a Coccidioides immitis antigen 2 domain that expresses B-cell-reactive epitopes. Infect. Immun. 65:3376-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zurlo, J., T. Crook, W. Green, J. Adams, C. Freer, J. Ratner, N. N"ikanatha, J. Rankin, L. Stetson, S. Yeager, and D. Pappagianis. 2000. Coccidioidomycosis in travelers returning from Mexico. Morb. Mortal. Wkly. Rep. 49:1004-1006. [PubMed] [Google Scholar]