Abstract
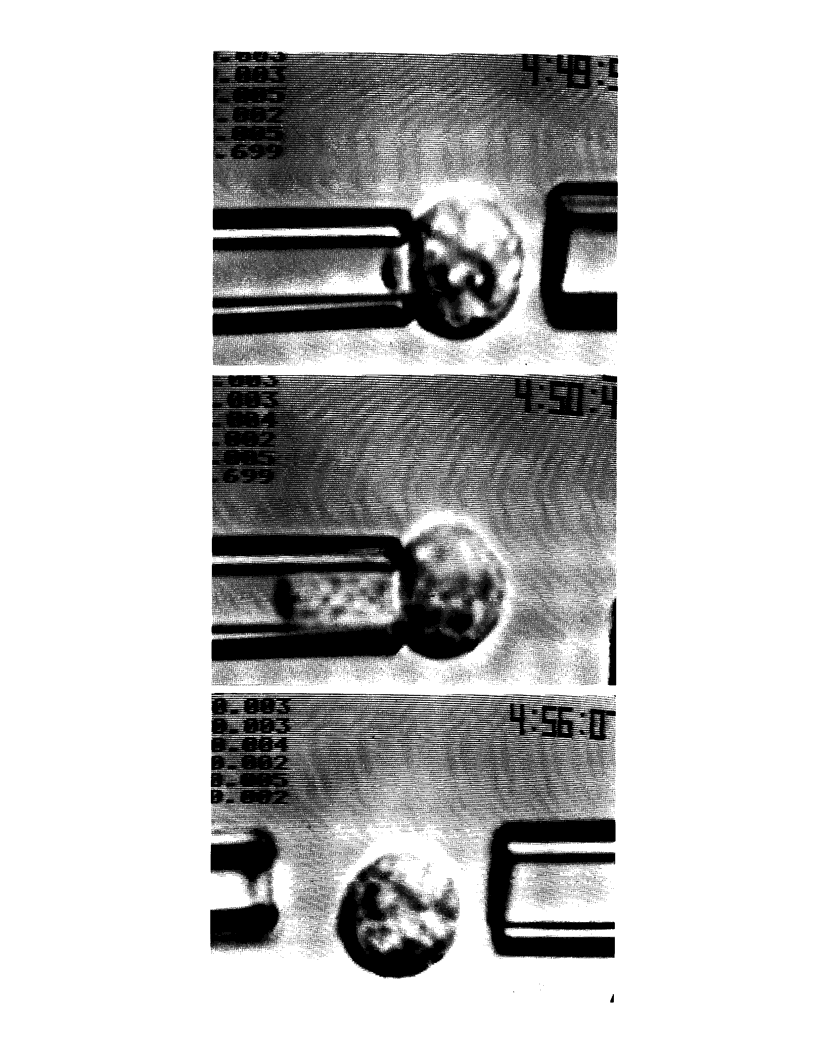
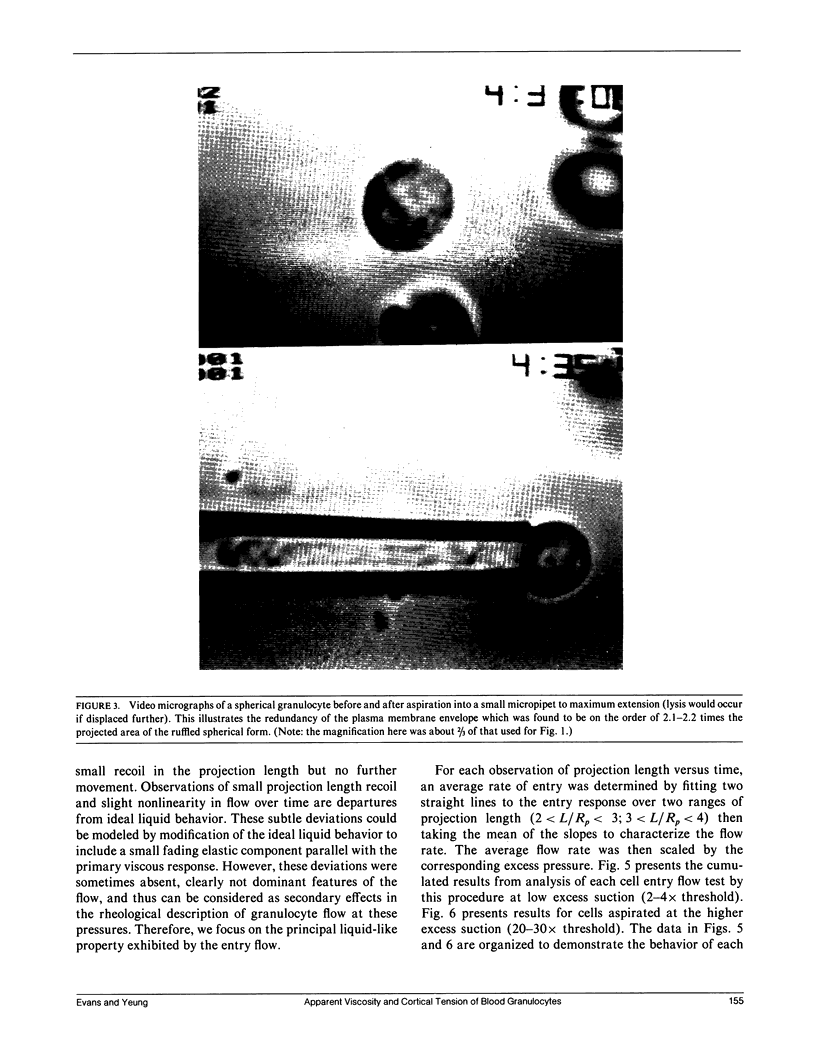
Continuous deformation and entry flow of single blood granulocytes into small caliber micropipets at various suction pressures have been studied to determine an apparent viscosity for the cell contents and to estimate the extent that dissipation in a cortical layer adjacent to the cell surface contributes to the total viscous flow resistance. Experiments were carried out with a wide range of pipet sizes (2.0-7.5 microns) and suction pressures (10(2)-10(4) dyn/cm2) to examine the details of the entry flow. The results show that the outer cortex of the cell maintains a small persistent tension of approximately 0.035 dyn/cm. The tension creates a threshold pressure below which the cell will not enter the pipet. The superficial plasma membrane of these cells appears to establish an upper limit to surface dilation which is reached after microscopic "ruffles" and "folds" have been pulled smooth. With aspiration of cells by small pipets (less than 2.7 microns), the limit to surface expansion was derived from the maximal extension of the cell into the pipet; final areas were measured to be 2.1 to 2.2 times the area of the initial spherical shape. For suctions in excess of a threshold, the response to constant pressure was continuous flow in proportion to excess pressure above the threshold with only a small nonlinearity over time until the cell completely entered the pipet (for pipet calibers greater than 2.7 microns). With a theoretical model introduced in a companion paper, (Yeung, A., and E. Evans., 1989, Biophys. J. 56:139-149) the entry flow response versus pipet size and suction pressure was analyzed to estimate the apparent viscosity of the cell interior and the ratio of cortical flow resistance to flow resistance from the cell interior. The apparent viscosity was found to depend strongly on temperature with values on the order of 2 x 10(3) poise at 23 degrees C, lower values of 1 x 10(3) poise at 37 degrees C, but extremely large values in excess of 10(4) poise below 10 degrees C. Because of scatter in cell response, it was not possible to accurately establish the characteristic ratio for flow resistance in the cortex to that inside the cell; however, the data showed that the cortex does not contribute significantly to the total flow resistance.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chien S., Schmid-Schönbein G. W., Sung K. L., Schmalzer E. A., Skalak R. Viscoelastic properties of leukocytes. Kroc Found Ser. 1984;16:19–51. [PubMed] [Google Scholar]
- Dong C., Skalak R., Sung K. L., Schmid-Schönbein G. W., Chien S. Passive deformation analysis of human leukocytes. J Biomech Eng. 1988 Feb;110(1):27–36. doi: 10.1115/1.3108402. [DOI] [PubMed] [Google Scholar]
- Evans E. A. Structural model for passive granulocyte behaviour based on mechanical deformation and recovery after deformation tests. Kroc Found Ser. 1984;16:53–71. [PubMed] [Google Scholar]
- Evans E., Kukan B. Passive material behavior of granulocytes based on large deformation and recovery after deformation tests. Blood. 1984 Nov;64(5):1028–1035. [PubMed] [Google Scholar]
- Gallin J. I. Human neutrophil heterogeneity exists, but is it meaningful? Blood. 1984 May;63(5):977–983. [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. Alteration of the cell periphery during granulocyte maturation: relationship to cell function. Blood. 1972 Mar;39(3):301–316. [PubMed] [Google Scholar]
- Miller M. E., Myers K. Cellular deformability of the human peripheral blood polymorphonuclear leukocyte: method of study, normal variation and effects of physical and chemical alterations. J Reticuloendothel Soc. 1975 Dec;18(6):337–345. [PubMed] [Google Scholar]
- Oliver J. M., Berlin R. D. Surface and cytoskeletal events regulating leukocyte membrane topography. Semin Hematol. 1983 Oct;20(4):282–304. [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Shih Y. Y., Chien S. Morphometry of human leukocytes. Blood. 1980 Nov;56(5):866–875. [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Sung K. L., Tözeren H., Skalak R., Chien S. Passive mechanical properties of human leukocytes. Biophys J. 1981 Oct;36(1):243–256. doi: 10.1016/S0006-3495(81)84726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick F. S., Stossel T. P. Contractile proteins in leukocyte function. Semin Hematol. 1983 Oct;20(4):305–321. [PubMed] [Google Scholar]
- Stossel T. P., Hartwig J. H., Yin H. L., Stendahl O. The motor of amoeboid leucocytes. Biochem Soc Symp. 1980;45:51–63. [PubMed] [Google Scholar]
- Sung K. L., Dong C., Schmid-Schönbein G. W., Chien S., Skalak R. Leukocyte relaxation properties. Biophys J. 1988 Aug;54(2):331–336. doi: 10.1016/S0006-3495(88)82963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung K. L., Schmid-Schönbein G. W., Skalak R., Schuessler G. B., Usami S., Chien S. Influence of physicochemical factors on rheology of human neutrophils. Biophys J. 1982 Jul;39(1):101–106. doi: 10.1016/S0006-3495(82)84495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valberg P. A., Albertini D. F. Cytoplasmic motions, rheology, and structure probed by a novel magnetic particle method. J Cell Biol. 1985 Jul;101(1):130–140. doi: 10.1083/jcb.101.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valberg P. A., Feldman H. A. Magnetic particle motions within living cells. Measurement of cytoplasmic viscosity and motile activity. Biophys J. 1987 Oct;52(4):551–561. doi: 10.1016/S0006-3495(87)83244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerius N. H., Stendahl O., Hartwig J. H., Stossel T. P. Distribution of actin-binding protein and myosin in polymorphonuclear leukocytes during locomotion and phagocytosis. Cell. 1981 Apr;24(1):195–202. doi: 10.1016/0092-8674(81)90515-8. [DOI] [PubMed] [Google Scholar]
- Yeung A., Evans E. Cortical shell-liquid core model for passive flow of liquid-like spherical cells into micropipets. Biophys J. 1989 Jul;56(1):139–149. doi: 10.1016/S0006-3495(89)82659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]




