Abstract
The genomes of three closely related bordetellae are currently being sequenced, thus providing an opportunity for comparative genomic approaches driven by an understanding of the comparative biology of these three bacteria. Although the other strains being sequenced are well studied, the strain of Bordetella parapertussis chosen for sequencing is a recent human clinical isolate (strain 12822) that has yet to be characterized in detail. This investigation reports the first phenotypic characterization of this strain, which will likely become the prototype for this species in comparison with the prototype strains of B. pertussis (Tohama I), B. bronchiseptica (RB50), and other isolates of B. parapertussis. Multiple in vitro and in vivo assays distinguished each species. B. parapertussis was more similar to B. bronchiseptica than to B. pertussis in many assays, including in BvgS signaling characteristics, presence of urease activity, regulation of urease expression by BvgAS, virulence in the respiratory tracts of immunocompromised mice, induction of anti-Bordetella antibodies, and serum antimicrobial resistance. In other assays, B. parapertussis was distinct from all other species (in pigment production) or more similar to B. pertussis (by lack of motility and cytotoxicity to a macrophage-like cell line). These results begin to provide phenotypes that can be related to genetic differences identified in the genomic sequences of bordetellae.
Bordetella pertussis, B. bronchiseptica, and B. parapertussis are gram-negative bacteria so closely related that they are considered subspecies (20, 26) and were recently described as the “classical” Bordetella (for a review, see reference 12). B. pertussis infects only humans, causing the acute respiratory disease known as whooping cough (pertussis) (5). B. bronchiseptica infects a wide variety of mammals and occasionally humans (14, 36). Although it frequently causes chronic asymptomatic infections (6), B. bronchiseptica can cause kennel cough in dogs, atrophic rhinitis in pigs, snuffles in rabbits, and bronchopneumonia in guinea pigs (13). B. parapertussis infects humans, causing a disease that is nearly indistinguishable from that caused by B. pertussis despite the failure of this organism to express pertussis toxin (18, 35). A bacterium also identified as B. parapertussis was isolated from the respiratory tracts of healthy sheep and sheep with chronic nonprogressive pneumonia (8, 28). Molecular phylogenetic analyses (28, 29, 33, 34, 38) found isolates from sheep (B. parapertussisov) to be distinct from those isolated from humans (B. parapertussishu) and B. bronchiseptica. These phylogenetic analyses suggest that B. parapertussishu, B. parapertussisov, and B. pertussis have each evolved independently from B. bronchiseptica (12, 33, 34). The host range, disease pathology, and ability to cause chronic or acute infection differ among the bordetellae (12), suggesting that informative phenotypes underlying these differences remain to be characterized.
The genomes of the RB50 strain of B. bronchiseptica (6), the Tohama I (5, 36) strain of B. pertussis (19), and the 12822 strain of B. parapertussishu are currently being sequenced by the Sanger Centre (http://www.sanger.ac.uk/Projects/Microbes). The 12822 strain of B. parapertussishu was isolated in Erlangen, Germany, during a prospective surveillance program (18). This strain was isolated from a nasopharyngeal swab taken from a 16-month-old boy by his pediatrician in July 1993. The boy had been coughing for 1 week, had no fever, and had not received any pertussis immunization. The cough was paroxysmal, and whooping was present, but there was no posttussive vomiting. His white blood cell count was normal (9,400 leukocytes/μl and a 59% lymphocyte component), and he recovered completely after coughing for a total of 5 weeks, as is typical of B. parapertussis infections. The disease caused by strain 12822 in this boy is characteristic of that caused by B. parapertussis in humans (18). This characteristic, along with the apparent high genetic homogeneity of isolates of B. parapertussishu (12, 38), makes strain 12822 a good representative strain for genomic and phenotypic investigations.
Phenotypes for the RB50 and Tohama I strains are well characterized, but the phenotypic characteristics for strain 12822 are not known. This investigation identifies the phenotypic similarities and differences between the 12822 strain of B. parapertussishu being sequenced and closely related strains of bordetellae under the conditions of in vitro culture, including phenotypic modulation, motility, pigment production, urease activity, serum antimicrobial killing, and macrophage toxicity, and in vivo respiratory infection of immunocompetent mice and immunodeficient mice. The results of this investigation indicate that B. parapertussishu shares more phenotypic similarities with B. bronchiseptica than it does with B. parapertussisov or B. pertussis. They also suggest that, among the classical species of Bordetella, phenotypic variation in host range, disease pathology, and process of infection is due to important differences in the presence and/or expression of only a small number of bacterial factors involved in host recognition and persistence.
MATERIALS AND METHODS
Strains and growth conditions.
A total of 170 and 10 isolates of B. parapertussis of human and ovine origin, respectively, were tested in this study. Most of the human isolates, including the 12822 strain of B. parapertussishu, were obtained from children with coughing illnesses in various regions of Germany between 1992 and 1997 (Pertussis Study Laboratory at the University Children and Adolescents Hospital, Erlangen, Germany). The others were isolated from humans with coughing illnesses in Finland (n = 10), France (n = 2), Italy (n = 10), The Netherlands (n = 9), Sweden (n = 18), and the United States (n = 4). Strains of B. parapertussisov were isolated from sheep in New Zealand (n = 5), such as Fr107, or Scotland (n = 5), such as H1. The Tohama I (strain 536), 18323 (ATCC 9797), GMT1, and CS strains of B. pertussis have been described previously (19, 22, 23). The RB50, RB53, RB54, GP1SN, and WD3 strains of B. bronchiseptica have been described previously (1, 6, 37).
All bordetellae were grown on Bordet-Gengou (BG) agar (Becton Dickinson Microbiology Systems) supplemented with 7.5% (for B. bronchiseptica and B. parapertussis) or 15% (for B. pertussis) defibrinated sheep blood. Motility assays were performed by stabbing colonies into Luria-Bertani or Stainer-Scholte (SS) medium containing soft agar (0.25 to 0.35% agar). Pigment production on tyrosine agar prepared as previously described (11, 28) and on BG-blood agar was examined in side-by-side comparisons. Nicotinic acid or MgSO4 was added to the various media to induce Bvg− modulation. Bacteria were incubated at 37°C.
Construction of Bvg−-phase-locked strains of B. parapertussis.
The ΔbvgS derivatives of the 12822 strain of B. parapertussishu and the H1 strain of B. parapertussisov were constructed using allelic exchange in a manner analogous to construction of the RB54 strain of B. bronchiseptica (6). Briefly, the bvgAS locus from the GP1 strain of B. bronchiseptica (1) was cloned into allelic exchange vector pEG25 (22) as a 5.2-kb EcoRI fragment. The 1.4-kb BglII-BclI fragment was then removed to delete amino acids 541 to 1000 spanning the second transmembrane domain, the linker, the transmitter, and most of the receiver domain of BvgS. This plasmid, pUH10, was used to replace the wild-type bvgAS loci of strains 12822 and H1 with the deletion mutation allele, resulting in the construction of strains 12822ΔbvgS and H1ΔbvgS, respectively. PCR was used to confirm the genetic organization of the bvgAS loci in both ΔbvgS strains. Both ΔbvgS strains formed large, flat, nonhemolytic colonies on BG-blood agar, which contained 40 mM MgSO4 or 20 mM nicotinic acid.
Assay for urease activity.
Strains of bordetellae were grown in Luria-Bertani or SS broth containing 0 or 40 mM MgSO4 and 0 or 10 mM urea as indicated (see Table 1). An aliquot of each culture (about 103 CFU) was plated onto BG-blood agar plates to detect the occurrence of spontaneous Bvg− mutants. Experiments in which Bvg− variants were detected were discarded. Bacteria used in the assay were collected by centrifugation of 500 μl of broth culture (optical density, 0.25), resuspended in 500 μl of urease test broth (85.6 mM NaCl, 14.7 mM KH2PO4, 33.3 mM urea, 13.2 μM phenol red), and incubated at 37°C for 24 h. The color change resulting from the increased pH due to the release of ammonia from urea was detected by measuring the absorbance at 560 nm.
TABLE 1.
General in vitro phenotypic differences between B. parapertussishu and closely related strains
| Species (origin) | Strain | Assay
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Motility | Pigment | Colony morphologya (nicotinic acid [mM])
|
Urease activityb (urea/MgSO4 [mM])
|
||||||||
| 0 | 2 | 4 | 8 | 0/0 | 10/0 | 0/40 | 10/40 | ||||
| B. parapertussis (human) | 12822, 1-169c | − | + | ++ | + | − | − | − | − | + | + |
| 12822ΔbvgS | − | + | − | − | − | − | + | + | + | + | |
| B. parapertussis (ovine; Scotland)d | H1, C | − | + | ++ | + | − | − | + | + | + | + |
| J1, G1, H1ΔbvgS | − | + | − | − | − | − | + | + | + | + | |
| K1 | − | − | − | − | − | − | + | + | + | + | |
| B. parapertussis (ovine; New Zealand) | Fr107-Fr111 | − | − | ++ | + | − | − | + | + | + | + |
| B. bronchiseptica (rabbit) | RB50 (Bvgwt)e | + | − | ++ | + | − | − | − | − | + | + |
| RB53 (Bvgc)f | − | − | ++ | ++ | ++ | ++ | − | − | − | − | |
| RB54 (Bvg−)g | + | − | − | − | − | − | + | + | + | + | |
| B. bronchiseptica (guinea pig) | GP1SN | + | − | ++ | + | − | − | − | − | + | + |
| B. pertussis (human) | Tohama I | − | − | ++ | ++ | ++ | + | − | − | − | − |
| GMT1 | − | − | ++ | + | − | − | − | − | − | − | |
| 18323 | − | − | ++ | + | − | − | − | − | − | − | |
| CS | − | − | ++ | ++ | ++ | − | − | − | − | − | |
++, small, domed colonies with a 7- to 10-mm hemolytic halo indicative of the Bvg+ phase; +, medium-sized, flat (ovoid) colonies with a 3- to 7-mm hemolytic halo indicative of a partial transition between phases; −, large, flat, no hemolytic halo indicative of the Bvg− phase.
+, high urease activity; −, very low or no urease activity. The isolates of B. parapertussishu tested for urease activity were 12822, 84099, 1, 11, RN130, 7254, 476, 89796, 11148, 36842, 13645, 133, and A-168.
1-169 are the number of isolates of B. parapertussishu tested.
B. parapertussisov isolates J1, G1, and K1 from Scotland appear to be Bvg− mutants.
Wild-type.
Constitutive Bvg+-phase-locked derivative of RB50.
Bvg−-phase-locked derivative of RB50.
ELISA, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblotting.
Titers of anti-Bordetella antibodies in serum samples collected 30 days after infection of mice with strain 12822, Fr107, RB50, or GMT1 were quantified by enzyme-linked immunosorbent assay (ELISA) as previously described (6) using whole cells from cultures of the respective bacterial strains. Mean titer values were compared by unpaired Student's t tests. Proteins in whole-cell extracts solubilized in sample buffer were separated on sodium dodecyl sulfate-polyacrylamide electrophoresis gels as described previously (6) and transferred to Immobilon-P (Millipore) membranes that were incubated with the respective anti-Bordetella mouse serum (1:2,000 dilution) to make immunoblots. Antigen-antibody complexes were detected with horseradish peroxidase-conjugated anti-mouse immunoglobulin G (Amersham) at a dilution of 1:2,500 and visualized by an enhanced chemiluminescence technique (Amersham).
Serum microbe killing and cytotoxicity assays.
Both serum microbe killing and cytotoxicity assays were performed as previously described (15). Briefly, serum was obtained from rabbits that were Bordetella free (naive) or immunized with heat-killed RB50, Fr107, 12822, or Tohama I. Bacteria (about 1,000 CFU/10 μl) in mid-log phase were mixed with the indicated serum (90 μl) or phosphate-buffered saline (PBS), incubated at 37°C for 1 h, and spread on BG agar for determination of bacterial survival. Cytotoxicity of bordetellae to the J774 macrophage cell line was determined at a multiplicity of infection of 10. Bacteria and J774 cells were incubated together at 37°C for 1 h, and the percentage of J774 cells killed was determined using the Cytotox96 kit (Promega). Mean values derived from both assays were compared by unpaired Student's t tests.
Experimental animals.
C57BL/6 mice were purchased from Charles River Laboratories. SCID-beige mice (BALB/c genetic background) were from facilities at the University of California at Los Angeles. All mice used were 4- to 6-week-old females. Inoculation of mice was performed as previously described (15, 17). Briefly, mice lightly sedated with halothane were inoculated with a high dose (5 × 104 CFU in 50 μl of PBS) of the designated Bordetella strain by pipetting the inoculum into the tips of the external nares. Groups of three mice were sacrificed at each time tested after inoculation. Blood was collected from mice by cardiac puncture. Colonization of the nasal cavity, trachea (1 cm), and lungs was quantified as previously described (15, 17). Animals were handled according to institutional guidelines. Colonization values are reported as means ± standard errors (SE) and compared by unpaired Student's t tests.
RESULTS AND DISCUSSION
Phenotypic modulation.
A hallmark of the bordetellae is phenotypic modulation controlled by the two-component BvgAS sensory transduction system in response to changes in environmental conditions (3, 21, 25). The response regulator (A) and sensor (S) components of this system are encoded in the bvg locus (4). Although Aricò et al. (4) reported the nucleotide and deduced amino acid sequences for this locus in multiple bordetellae, the strains sequenced were not the strains whose genomes are being sequenced by the Sanger Centre. Therefore, these sequences for the bvgAS components in strains Tohama I (strain 536), 12822, and RB50 were compared to each other and that of the 165 strain of B. pertussis reported by Aricò et al. (4) (accession number M25401 in the EMBL-GenBank-DDBJ nucleotide sequence data libraries). The 0.63-kb nucleotide sequence for bvgA from strain 165 was identical to that from Tohama I (strain 536), which was 99% identical to that from 12822 and 99.4% identical to that from RB50. This sequence from strain 12822 was 99.7% identical to that from RB50. The deduced 219-amino-acid sequence for BvgA was identical for all three species.
The 3.717-kb nucleotide sequence for bvgS from strain 165 was 99.9% identical to that from Tohama I (strain 536), and both strains of B. pertussis were 95.3% identical to those from strains 12822 and RB50. This sequence from 12822 was 99.5% identical to that from RB50. The deduced 1,238-amino-acid sequence for BvgS from 165 was 99.8% identical to that from Tohama I (strain 536), and both strains of B. pertussis were 95.2% identical to those from 12822 and RB50. This sequence from 12822 was 99.5% identical to that from RB50. As reported by Aricò et al. (4), most of the base pair (67%) and amino acid (68%) differences among the bvgS sequences compared occurred in the predicted periplasmic regions of BvgS. This region from 165 was identical in amino acid sequence to that from Tohama I (strain 536), and both strains of B. pertussis were 92% identical in amino acid sequence to those from 12822 and RB50. Interestingly, the amino acid sequence for the periplasmic region of strain 12822 was identical to that of RB50. These similarities and differences in sequences are consistent with phylogenetic analyses (33, 34, 38), suggesting that B. parapertussis is more closely related to B. bronchiseptica than to B. pertussis.
The BvgAS system mediates the transition between virulent (Bvg+), intermediate, and avirulent (Bvg−) phases (7, 9). When grown at 37°C in the absence of chemical modulators such as nicotinic acid or MgSO4, classical bordetellae are in the Bvg+ phase, in which BvgAS activates the expression of virulence factors and represses motility and virulence-repressed genes. As the temperature is decreased below 37°C or the level of chemical modulator is increased, conditions become semimodulating and classical bordetellae enter the intermediate (Bvgi) phase, where BvgAS activates factors that are expressed exclusively in this phase and only a subset of Bvg+-phase factors (9, 31). Further decreases in temperature or increases in the level of chemical modulator induced bordetellae to enter the Bvg− phase, where BvgAS is inactive and no longer activates expression of virulence factors nor represses motility and virulence-repressed genes. Various in vitro phenotypes, such as colony morphology, motility, pigment production, and urease activity, are used to distinguish the bordetellae, characterize the Bvg phase, or both. Therefore, Bvg control of these in vitro phenotypes for the 12822 strain of B. parapertussishu was determined and compared to those for B. bronchiseptica and B. pertussis in order to identify phenotypic similarities.
Expression of flagella and motility in soft agar under Bvg−-phase conditions was previously observed for B. bronchiseptica but not B. pertussis (1, 2). The observed lack of motility by strains of B. parapertussis has been previously reported (1, 13, 28) and recently reviewed with that known for other bordetellae (12), but conditions for growth of the bacteria prior to and during the motility assay were not clearly described in any of these studies. Therefore, the possibility that, like B. bronchiseptica, B. parapertussis is motile only under modulating Bvg−-phase conditions was examined. Under no conditions were the isolates of B. parapertussis tested found to be motile (Table 1), including isolate K1, which was previously reported to be motile (28). All strains of B. bronchiseptica tested were motile in the presence of nicotinic acid, MgSO4, or low temperature. Under no conditions was motility observed in the strains of B. pertussis (Table 1) tested and the Bvg−-phase-locked derivatives (12822ΔbvgS and H1ΔbvgS) of B. parapertussis. Results presented here show that, like B. pertussis (1, 2) and unlike B. bronchiseptica, B. parapertussis is not motile, even under Bvg−-phase conditions.
Production of brown pigment on tyrosine agar, which is attributed to a tyrosinase that converts tyrosine into a melanin-like pigment (11), is a phenotype expressed by many strains of B. parapertussis but not by other bordetellae (11, 28). In this investigation, pigment production on tyrosine agar and BG-blood agar by all isolates of bordetellae tested was examined under Bvg+- or Bvg−-phase conditions. Brown pigment was produced under all conditions tested by all 170 isolates of B. parapertussishu, including strain 12822 (Table 1). This pigment was also produced by the isolates of B. parapertussisov from Scotland, except for K1, which was previously reported to not produce pigment (28). In contrast, none of the other isolates of B. parapertussisov from New Zealand, B. bronchiseptica, or B. pertussis that were assessed produced pigment under any condition tested. Pigment production is not regulated by BvgAS in the bordetellae and appears to be a conserved phenotype in only B. parapertussishu.
Hemolytic activity and colony morphology on blood agar have been shown (22) to be accurate indicators of the sensitivity of BvgAS to signal the Bvg− to Bvg+ transition in response to decreasing chemical modulator and increasing temperature. However, the BvgS signaling characteristics of B. parapertussis have not been previously compared to other classical bordetellae. As previously described (22), the strains of B. bronchiseptica tested did not vary in sensitivity to nicotinic acid concentration whereas the strains of B. pertussis tested did vary in sensitivity to the modulator tested (Table 1). Uniform sensitivity to relatively low concentrations of nicotinic acid was observed for strain 12822 and the other 169 isolates of B. parapertussishu tested, suggesting that B. parapertussishu displays BvgAS signaling characteristics similar to that of B. bronchiseptica. Isolates of B. parapertussisov that are not spontaneously occurring Bvg−-phase variants (isolates J1, G1, and K1) also display BvgAS signaling characteristics similar to that of B. bronchiseptica. The periplasmic domain of BvgS was previously shown to be responsible for differences in signal sensitivity (22). Most of the variation in bvgS nucleotide sequences and BvgS amino acid sequences among the three species coincides with the periplasmic domain (4). Interestingly, both the signaling sensitivity and the amino acid sequence of the periplasmic domain of BvgS from B. parapertussis are more similar to those from B. bronchiseptica than to those from B. pertussis.
Urease activity differentiates the urease-negative B. pertussis strains from the urease-positive B. parapertussis and B. bronchiseptica strains. Like that of other strains of B. bronchiseptica (24), urease activity in the RB50 strain of B. bronchiseptica and all isolates of B. parapertussishu tested here was very low under Bvg+-phase conditions but high under modulating Bvg−-phase conditions (Table 1). Regulation of urease activity by BvgAS was demonstrated in the RB50 and 12822 strains, whose ΔbvgS derivatives produced urease activity after growth under modulating and nonmodulating conditions, while no urease activity was detected in a Bvg+ constitutive derivative of RB50 (RB53) grown under either growth condition. In contrast, all isolates of B. parapertussisov tested, including the ΔbvgS derivative of strain H1, constitutively expressed urease activity under either growth condition (Table 1). The presence of 10 mM urea in the growth medium had no influence on urease production by any of the strains tested. Control of urease expression by BvgAS, therefore, represents a phenotype shared by B. bronchiseptica and B. parapertussishu but not by the other bordetellae tested.
Respiratory tract colonization in immunocompetent mice.
A previous study (15) uncovered differences between the abilities of the RB50 strain of B. bronchiseptica and the Tohama I strain of B. pertussis to grow in the respiratory tracts of mice. The 12822 strain of B. parapertussishu, however, has not been analyzed in vivo. Therefore, a high-dose regimen was used to examine the abilities of strain 12822 and other classical bordetellae to colonize the respiratory tracts of C57BL/6 mice (Fig. 1). Five days after inoculation with RB50 or 12822, levels of colonization in the nasal cavity, trachea, and lungs were similar (P ≥ 0.2) for both strains. These initial levels of colonization for RB50 and 12822 were greater than (P ≤ 0.001) that of the Fr107 strain of B. parapertussisov in all sites, with the lower respiratory tract of these mice being poorly colonized by B. parapertussisov. Similar to previously reported (17) colonization results for human-adapted strains of B. pertussis (Tohama I) and B. parapertussis (CN2591), 12822 efficiently colonized the entire respiratory tracts of immunocompetent mice.
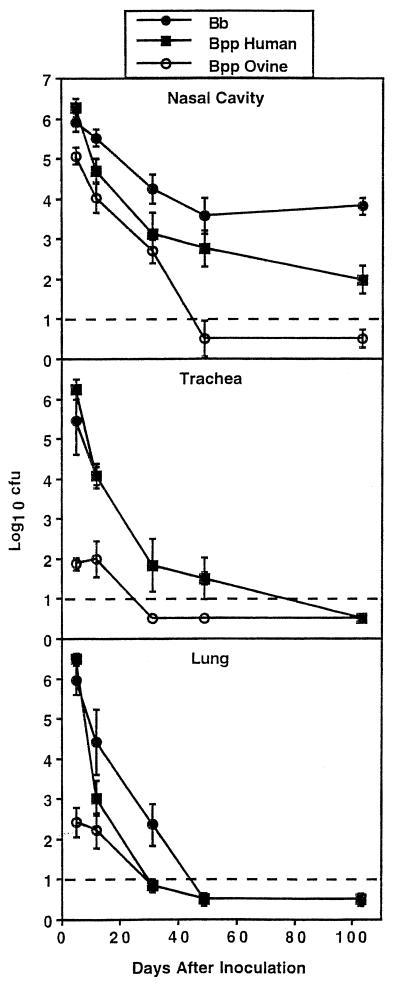
FIG. 1.
Time course for respiratory tract colonization in C57BL/6 mice by the RB50 strain of B. bronchiseptica (Bb), the 12822 strain of B. parapertussishu (Bpp Human), or the FR107 strain of B. parapertussisov (Bpp Ovine). Mice were inoculated intranasally with a high dose (50 μl of PBS containing 5 × 104 CFU) of the indicated strains. At the indicated times after inoculation, three to six mice were sacrificed and the number of CFU recovered from the nasal cavity, trachea, and lungs was determined. The broken line indicates the limit of detection or 10 CFU. Points represent means ± SE of the log10 transformation of the CFU recovered from each mouse.
The persistence of these strains varied with the site in the respiratory tract tested (Fig. 1). Although RB50 persisted in greater numbers (P ≤ 0.01) in the nasal cavity than 12822, both RB50 and 12822, but not FR107, persisted in the nasal cavity throughout the experimental period of 103 days. Persistence of both RB50 and 12822 in the trachea was similar (>50 days after inoculation; P ≥ 0.7) and longer (P ≤ 0.001) than that of Fr107 (<30 days after inoculation). Both 12822 and Fr107 were cleared from the lungs faster (31 days after inoculation) than RB50 (49 days after inoculation). Using BALB/c mice and a similar inoculation regimen, Harvill et al. (17) observed clearance of Tohama I and CN2591 by 28 days after inoculation in the nasal cavity and by 21 days after inoculation in the trachea and lungs whereas RB50 persisted in the nasal cavity throughout the experimental period of 50 days and was not cleared from the trachea and lungs until 50 days after inoculation. These results indicate that the ability to persist in the respiratory tracts of mice is not a common phenotype of B. pertussis and B. parapertussis, whereas this ability is a phenotype common to strains of B. bronchiseptica.
The abilities of additional isolates (11867, 18763, 133, 11148, 36842, 9100436, 11, A-002, A-168, and 1) of B. parapertussishu to colonize C57BL/6 mice (one mouse per strain) 5 days after inoculation with a high-dose inoculation regimen were tested to determine if their colonization ability differed from that for strain 12822. Colonization (log10 range) of the nasal cavity, trachea, and lungs by all additional human isolates tested (5.5 to 6.2, 5.0 to 5.9, and 5.3 to 6.4 CFU, respectively) overlapped with that for 12822 (6.0 to 6.4, 5.8 to 6.2, and 6.4to 6.6 CFU, respectively), suggesting that all isolates of B. parapertussis tested behave similarly in mice. This behavior in mice is consistent with the apparent high genetic homogeneity of isolates of B. parapertussishu (38).
Serum antibody responses.
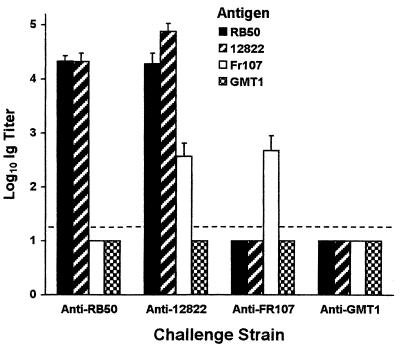
A previous study (15) uncovered a vigorous antibody response in mice after infection with the RB50 strain of B. bronchiseptica and little antibody response after infection with the Tohama I strain of B. pertussis. Therefore, the antibody response of mice to the 12822 strain of B. parapertussishu administered by the intranasal route was compared with this response to other classical bordetellae (Fig. 2) by ELISAs with the respective strain as antigen. Serum samples from mice 30 days after inoculation with 12822 contained similarly high titers (P ≥ 0.2) of anti-12822 antibodies that recognized 12822 and RB50 but lower titers (P ≤ 0.02) of antibodies that recognized the Fr107 strain of B. parapertussisov. Serum samples from mice 30 days after inoculation with RB50 contained similarly high titers (P ≥ 0.4) of anti-RB50 antibodies that recognized only RB50 and 12822. In contrast, anti-Bordetella antibodies could not be detected above background in serum samples from mice 30 days after inoculation with the GMT1 strain of B. pertussis, which was consistent with previous observations (15) of Tohama I. Serum samples from mice 30 days after inoculation with Fr107 contained intermediate titers of anti-Fr107 antibodies that recognized Fr107 but not GMT1, RB50, or 12822. In addition, serum samples from mice infected with 12822, Fr107, or RB50 did not recognize GMT1 above background (Fig. 2). Western blots used to visualize the repertoire of Bordetella antigens mimicked the trends observed by ELISA (data not shown). These results demonstrate that induction of a strong antibody response during colonization of the mouse respiratory tract is shared by B. bronchiseptica and B. parapertussishu but greatly diminished in or absent from B. pertussis and B. parapertussisov.
FIG. 2.
Comparison of anti-Bordetella antibody titers in serum samples collected 30 days after intranasal challenge or infection of mice with a high dose (50 μl of PBS containing 5 × 104 CFU) of the RB50 strain of B. bronchiseptica, the 12822 strain of B. parapertussishu, the FR107 strain of B. parapertussisov, or the GMT1 strain of B. pertussis. Whole cells of the indicated strain were used as the antigen in each ELISA. The secondary antibody used detected the immunoglobulin of all isotypes. Bars represent means ± SE (n = 5) of the log10 transformation of the immunoglobulin titer detected. The broken line indicates the limit of detection.
Serum antimicrobial resistance.
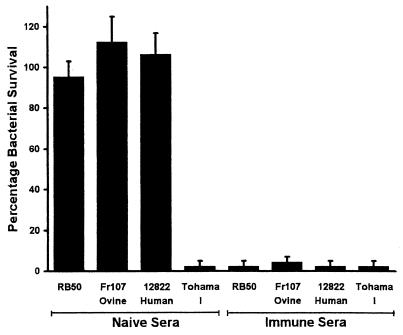
Previous studies (15, 17) found the RB50 strain of B. bronchiseptica, but not the Tohama I strain of B. pertussis, to be resistant to the innate antimicrobial activities of naive serum. Therefore, the resistance of the 12822 strain of B. parapertussishu in serum was compared with that to other bordetellae (Fig. 3). The high levels of resistance of 12822 and Fr107 were similar (P ≥ 0.4) to that of RB50 in naive serum. The Tohama I strain of B. pertussis was killed (>95%) by naive serum. None of the bordetellae tested were resistant to immune serum. These results suggest that, unlike B. pertussis, both B. bronchiseptica and B. parapertussis have the phenotypic ability to survive the host's innate antimicrobial agents present in blood and lymph fluids.
FIG. 3.
Serum antimicrobial resistance of the RB50 strain of B. bronchiseptica, the FR107 strain of B. parapertussisov, the 12822 strain of B. parapertussishu, and the Tohama I strain of B. pertussis. Bacteria were grown to mid-log phase in SS broth and diluted in PBS so that a total of 1,000 bacteria were incubated at 37°C for 1 h in 100 μl of 90% serum obtained from rabbits that were Bordetella free (naive) or immunized with heat-killed RB50, Fr107, 12822, or Tohama I. Bars represent means ± SE (n = 3).
Virulence in immunodeficient mice.
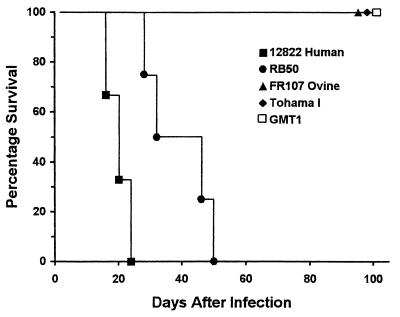
SCID-beige mice, which are deficient in B cells, T cells, and natural killer cells (10, 30), were used to compare the ability of the 12822 strain of B. parapertussishu to overcome innate immunity with that of other classical bordetellae (Fig. 4). These mice, inoculated with a high dose of either strain of B. pertussis tested (Tohama I or GMT1) or the Fr107 strain of B. parapertussisov, showed no signs of illness throughout the experimental period of 103 days. In contrast, all SCID-beige mice inoculated with 12822 or the RB50 strain of B. bronchiseptica succumbed to lethal infection by 24 or 50 days after inoculation, respectively. All strains tested were recovered from the lungs of mice that survived to the end of the experimental period, indicating that they shared the ability to persist in the lower respiratory tract. The observation that B. parapertussishu was highly virulent in these mice indicates that the B-cell and T-cell components of adaptive immunity are required to limit infection by B. parapertussishu, as previously shown with B. bronchiseptica (15, 17). Immune mechanisms still active in these mice are able to control B. parapertussisov, as previously shown with B. pertussis (15, 17).
FIG. 4.
Survival of SCID-beige mice inoculated with the 12822 strain of B. parapertussishu (filled square), the RB50 strain of B. bronchiseptica (filled circle), the FR107 strain of B. parapertussisov (filled triangle), or the Tohama I (filled diamond) or GMT1 (open square) strains of B. pertussis. Groups of four mice were inoculated intranasally with a high dose (50 μl of PBS containing 5 × 104 CFU) of the indicated strains.
The virulence of B. bronchiseptica in SCID-beige mice was previously shown to require expression of adenylate cyclase toxin (16). Since all of the bordetellae tested here express this toxin, there is at least one other factor unique to B. bronchiseptica and B. parapertussishu that is also required for virulence in these immunodeficient mice. Interestingly, B. parapertussishu, B. parapertussisov, and B. bronchiseptica are highly resistant in vitro to the antimicrobial components in naive serum, whereas B. pertussis was killed (Fig. 3). Lipopolysaccharides (LPS) are known to influence serum antimicrobial resistance in other bacteria, and the LPS structures vary among bordetellae (12, 27). In addition, the LPS structures of B. bronchiseptica and B. pertussis correlate with their respective serum antimicrobial resistance phenotypes (32). The presence of the membrane distal polysaccharide domains on the LPS of B. bronchiseptica, B. pertussis, and B. parapertussishu is essential for the expression of full virulence in immunocompetent (BALB/c) and immunodeficient (SCID-beige) mice (17). Furthermore, the presence of the O-antigen-like repeat in the distal polysaccharide domain of the LPS from B. bronchiseptica and B. parapertussishu is required for both survival in naive serum and virulence in SCID mice (17; V. C. Burns and E. T. Harvill, unpublished results). The wbm genes are required for assembly of O-antigen structures in B. bronchiseptica and B. parapertussishu, but these genes are not present in B. pertussis, which does not survive in naive serum and is avirulent in SCID mice (Fig. 3). Although B. parapertussisov has the wbm genes and is resistant to naive serum, it is avirulent in SCID mice, suggesting that it lacks some other factor required for virulence. Together, these results suggest that the different LPS structures on these closely related bordetellae influence their differing levels of resistance in serum and virulence in immunocompetent and immunodeficient mice.
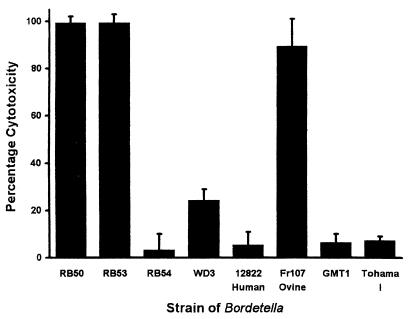
Macrophage cytotoxicity.
Previous studies (15, 17, 37) found B. bronchiseptica, but not B. pertussis, to be cytotoxic to the mouse macrophage-like cell line J774. Therefore, the cytotoxicity of the 12822 strain of B. parapertussishu was compared with that of other bordetellae (Fig. 5). Like both B. pertussis strains tested (Tohama I and GMT1), strain 12822 was minimally cytotoxic to J774 cells, whereas the RB50 strain of B. bronchiseptica and the Fr107 strain of B. parapertussisov were highly cytotoxic. The Bvg+-phase-locked derivative of RB50, strain RB53, was highly cytotoxic, whereas the Bvg−-phase-locked derivative of RB50, strain RB54, was minimally cytotoxic, indicating that cytotoxicity is regulated by BvgAS, as previously shown (15). The WD3 derivative of RB50 contains an in-frame deletion mutation of bscN (ΔbscN), which is a putative ATPase required by the type III secretion (TTS) system for protein export (37). Cytotoxicity of WD3 was about 25% (P ≤ 0.001) of that of RB50, RB53, and FR107 but still greater (P ≤ 0.03) than that of both strains of B. pertussis tested, RB54 and 12822.
FIG. 5.
Cytotoxicity of Bordetella strains to the mouse macrophage-like cell line J774. Bacteria were added at a multiplicity of infection of 10 to J774 cells in culture medium in a 96-well plate. The plate was spun at 500 × g for 10 min and then incubated at 37°C for 4 h. Cytotoxicity was assessed using the Cytotox96 kit according to the manufacturer's instructions. Bacteria tested were the RB50 strain of B. bronchiseptica; its Bvg+ and Bvg− derivatives (RB53 and RB54, respectively); its ΔbscN derivative (WD3), whose phenotype is TTS deficient; and the 12822 strain of B. parapertussishu, the FR107 strain of B. parapertussisov, and the GMT1 and Tohama I strains of B. pertussis. Bars represent means ± SE (n = 3) of the percentages of total lysis by detergent.
All bordetellae studied in this investigation have genes encoding a TTS system, but expression has only been detected in B. bronchiseptica and B. parapertussisov (37). As previously shown (37, 39), the cytotoxicity of B. bronchiseptica to the macrophage-like cell line J774 is much reduced by deletion of bscN or deletion of bvgAS, which is required for expression of bscN. Interestingly, the relative differences in infection pattern, virulence, and serum antimicrobial resistance observed among the classical bordetellae tested in this investigation did not correlate with their observed cytotoxicity in vitro. Cytotoxicity did, however, correlate with the previously observed expression of a functional TTS system in B. bronchiseptica and transcription of bscN in B. parapertussisov, suggesting that TTS is involved in cytotoxicity. In comparison, transcription of TTS genes was not detected (37) in B. parapertussishu or the strains of B. pertussis tested in this investigation, and these strains, like the TTS mutant of B. bronchiseptica, are not cytotoxic.
This investigation prompts speculation on the evolution of host range (see also reference 12). The ability of B. parapertussishu to establish infections in mice that are comparable to those by B. bronchiseptica suggests that this bacterium has not lost the ability to infect nonhuman animals. Their clinical isolation exclusively from humans could reflect sampling and identification biases, i.e., B. parapertussishu, may routinely infect animals in which respiratory infections are not monitored or from which bordetellae are not identifiable by standard methods. Alternatively, B. parapertussishu may in fact be limited in host range to humans, but this limitation may result from constraints due to transmissibility or host availability rather than to its absolute ability to colonize the respiratory tracts of nonhuman hosts. As sequences for the Bordetella genomes become available, comparative genome-based approaches, such as DNA microarrays, will reveal those bacterial (12) and host genes differentially expressed among the bordetellae during an infection. Testing of these genes by the assays described here is an approach for relating individual genes to species-specific Bordetella phenotypes that will lead to a better understanding of the evolution of host range and the molecular basis of Bordetella pathogenesis.
Acknowledgments
We are grateful to the following colleagues for providing isolates of B. parapertussis: E. Falsen (Goeteborg, Sweden), N. Guiso (Paris, France), H. Hallander (Stockholm, Sweden), J. Hunter (Palmerston North, New Zealand), P. Mastrantonio (Rome, Italy), J. Mertsola (Turku, Finland), F. Mooi (Bilthoven, The Netherlands), J. Porter (Edinburgh, Scotland), and R. Weyant (Atlanta, Ga.). Special thanks go to Jim Cherry, who first suggested the phenotypic analysis of B. parapertussis.
This work was supported by a European Society for Pediatric Infectious Diseases (ESPID) Fellowship Award to U.H., a Damon Runyon-Walter Winchell Foundation postdoctoral fellowship to M.H.Y., Gobierno de Navarra and HOECHST-Sociedad Espanola de Enfermedades Infecciosas y Microbiologia Clinica fellowships and a postdoctoral fellowship from Universidad de Navarra (PIUNA) (to G.M.D.T.), NIH grants AI38417 (to J.F.M.) and AI43986 (to P.A.C), and USDA grants 1999-02298 (to J.F.M.) and 960-1856 (to E.T.H.).
Editor: D. L. Burns
REFERENCES
- 1.Akerley, B. J., and J. F. Miller. 1993. Flagellin gene transcription in Bordetella bronchiseptica is regulated by the BvgAS virulence control system. J. Bacteriol. 175:3468-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akerley, B. J., D. M. Monack, S. Falkow, and J. F. Miller. 1992. The bvgAS locus negatively controls motility and synthesis of flagella in Bordetella bronchiseptica. J. Bacteriol. 174:980-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aricò, B., J. F. Miller, C. Roy, S. Stibitz, D. Monack, S. Falkow, R. Gross, and R. Rappuoli. 1989. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc. Natl. Acad. Sci. USA 86:6671-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aricò, B., V. Scarlato, D. M. Monack, S. Falkow, and R. Rappuoli. 1991. Structural and genetic analysis of the bvg locus in Bordetella species. Mol. Microbiol. 5:2481-2491. [DOI] [PubMed] [Google Scholar]
- 5.Cherry, J. D., and U. Heininger. 1998. Pertussis, p. 1423-1440. In R. D. Feigin and J. D. Cherry (ed.), Textbook of pediatric infectious diseases. W. B. Saunders, Philadelphia, Pa.
- 6.Cotter, P. A., and J. F. Miller. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 62:3381-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter, P. A., and J. F. Miller. 2001. Bordetella, p. 620-674. In E. Groisman (ed.), Principles of bacterial pathogenesis. Academic Press, San Diego, Calif.
- 8.Cullinane, L. C., M. R. Alley, R. B. Marshall, and B. W. Manktelow. 1987. Bordetella parapertussis from lambs. N. Z. Vet. J. 35:175.. [DOI] [PubMed] [Google Scholar]
- 9.Deora, R., H. J. Bootsma, J. F. Miller, and P. A. Cotter. 2001. Diversity in the Bordetella virulence regulon: transcriptional control of a Bvg-intermediate phase gene. Mol. Microbiol. 40:669-683. [DOI] [PubMed] [Google Scholar]
- 10.Dorshkind, K., G. M. Keller, R. A. Phillips, R. G. Miller, G. C. Bosma, M. O'Toole, and M. J. Bosma. 1984. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J. Immunol. 132:1804-1808. [PubMed] [Google Scholar]
- 11.Ensminger, P. W. 1953. Pigment production by Haemophilus parapertussis. J. Bacteriol. 65:509-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerlach, G., F. von Wintzingerode, B. Middendorf, and R. Gross. 2001. Evolutionary trends in the genus Bordetella. Microbes Infect. 3:61-72. [DOI] [PubMed] [Google Scholar]
- 13.Goodnow, R. A. 1980. Biology of Bordetella bronchiseptica. Microbiol. Rev. 44:722-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gueirard, P., C. Weber, A. Le Coustumier, and N. Guiso. 1995. Human Bordetella bronchiseptica infection related to contact with infected animals: persistence of bacteria in host. J. Clin. Microbiol. 33:2002-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvill, E. T., P. A. Cotter, and J. F. Miller. 1999. Pregenomic comparative analysis between Bordetella bronchiseptica RB50 and Bordetella pertussis Tohama I in murine models of respiratory tract infection. Infect. Immun. 67:6109-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvill, E. T., P. A. Cotter, M. H. Yuk, and J. F. Miller. 1999. Probing the function of Bordetella bronchiseptica adenylate cyclase toxin by manipulating host immunity. Infect. Immun. 67:1493-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvill, E. T., A. Preston, P. A. Cotter, A. G. Allen, D. J. Maskell, and J. F. Miller. 2000. Multiple roles for Bordetella lipopolysaccharide molecules during respiratory tract infection. Infect. Immun. 68:6720-6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heininger, U., K. Stehr, S. Schmitt-Grohé, C. Lorenz, R. Rost, P. D. Christenson, M. Überall, and J. D. Cherry. 1994. Clinical characteristics of illness caused by Bordetella parapertussis compared with illness caused by Bordetella pertussis. Pediatr. Infect. Dis. J. 13:306-309. [DOI] [PubMed] [Google Scholar]
- 19.Kasuga, T., Y. Nakase, K. Ukishima, and K. Takatsu. 1954. Studies on Haemophilus pertussis. Part III. Some properties of each phase of H. pertussis. Kitasato Arch. Exp. Med. 27:37-48. [PubMed] [Google Scholar]
- 20.Kloos, W. E., N. Mohapatra, W. J. Dobrogosz, J. W. Ezzell, and C. R. Manclark. 1981. Deoxyribonucleotide sequence relationships among Bordetella species. Int. J. Syst. Bacteriol. 31:173-176. [Google Scholar]
- 21.Lacey, B. W. 1960. Antigenic modulation of Bordetella pertussis. J. Hyg. 58:57-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez de Tejada, G., J. F. Miller, and P. A. Cotter. 1996. Comparative analysis of the virulence control systems of Bordetella pertussis and Bordetella bronchiseptica. Mol. Microbiol. 22:895-908. [DOI] [PubMed] [Google Scholar]
- 23.Martinez de Tejada, G., P. A. Cotter, U. Heininger, A. Camilli, B. J. Akerley, J. J. Mekalanos, and J. F. Miller. 1998. Neither the Bvg− phase nor vrg6 locus of Bordetella pertussis is required for respiratory infection in mice. Infect. Immun. 66:2762-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMillan, D. J., M. Shojaei, G. S. Chhatwal, C. A. Guzmán, and M. J. Walker. 1996. Molecular analysis of the bvg-repressed urease of Bordetella bronchiseptica. Microb. Pathog. 21:379-394. [DOI] [PubMed] [Google Scholar]
- 25.Miller, J. F., S. A. Johnson, W. J. Black, D. T. Beattie, J. J. Mekalanos, and S. Falkow. 1992. Constitutive sensory transduction mutations in the Bordetella pertussis bvgS gene. J. Bacteriol. 174:970-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musser, J. M., E. L. Hewlett, M. S. Peppler, and R. K. Selander. 1986. Genetic diversity and relationships in populations of Bordetella spp. J. Bacteriol. 166:230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preston, A., A. G. Allen, J. Cadisch, R. Thomas, K. Stevens, C. M. Churcher, K. L. Badcock, J. Parkhill, B. Barrell, and D. J. Maskell. 1999. Genetic basis for lipopolysaccharide O-antigen biosynthesis in bordetallae. Infect. Immun. 67:3763-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porter, J. F., K. Connor, and W. Donachie. 1994. Isolation and characterization of Bordetella parapertussis-like bacteria from ovine lungs. Microbiology 140:255-261. [DOI] [PubMed] [Google Scholar]
- 29.Porter, J. F., K. Connor, and W. Donachie. 1996. Differentiation between human and ovine isolates of Bordetella parapertussis using pulsed-field gel electrophoresis. FEMS Microbiol. Lett. 135:131-135. [DOI] [PubMed] [Google Scholar]
- 30.Roder, J., and A. Duwe. 1979. The beige mutation in the mouse selectively impairs natural killer cell function. Nature 278:451-453. [DOI] [PubMed] [Google Scholar]
- 31.Stockbauer, K. E., B. Fuchslocher, J. F. Miller, and P. A. Cotter. 2001. Identification and characterization of BipA, a Bordetella Bvg-intermediate phase protein. Mol. Microbiol. 39:65-78. [DOI] [PubMed] [Google Scholar]
- 32.van den Akker, W. M. 1998. Lipopolysaccharide expression within the genus Bordetella: influence of temperature and phase variation. Microbiology 144:1527-1535. [DOI] [PubMed] [Google Scholar]
- 33.van der Zee, A., H. Groenendijk, M. Peeters, and F. R. Mooi. 1996. The differentiation of Bordetella parapertussis and Bordetella bronchiseptica from humans and animals as determined by DNA polymorphism mediated by two different insertion sequence elements suggests their phylogenetic relationship. Int. J. Syst. Bacteriol. 46:640-647. [DOI] [PubMed] [Google Scholar]
- 34.van der Zee, A., F. Mooi, J. Van Embden, and J. Musser. 1997. Molecular evolution and host adaptation of Bordetella spp.: phylogenetic analysis using multilocus enzyme electrophoresis and typing with three insertion sequences. J. Bacteriol. 179:6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wirsing von Konig, C. H., and H. Finger. 1994. Role of pertussis toxin in causing symptoms of Bordetella parapertussis infection. Eur. J. Clin. Microbiol. Infect. Dis. 13:455-458. [DOI] [PubMed] [Google Scholar]
- 36.Woolfrey, B. F., and J. A. Moody. 1991. Human infections associated with Bordetella bronchiseptica. Clin. Microbiol. Rev. 4:243-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuk, M. H., E. T. Harvill, and J. F. Miller. 1998. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol. Microbiol. 28:945-959. [DOI] [PubMed] [Google Scholar]
- 38.Yuk, M. H., U. Heininger, G. Martinez de Tejada, and J. F. Miller. 1998. Human but not ovine isolates of Bordetella parapertussis are highly clonal as determined by PCR-based RAPD fingerprinting. Infection 26:270-273. [DOI] [PubMed] [Google Scholar]
- 39.Yuk, M. H., E. T. Harvill, P. A. Cotter, and J. F. Miller. 2000. Modulation of host immune responses, induction of apoptosis and inhibition of NF-κB activation by the Bordetella type III secretion system. Mol. Microbiol. 35:991-1004. [DOI] [PubMed] [Google Scholar]