Abstract
Based on the existence of ATP-sensitive potassium channels in the plasma membrane of pancreatic beta cells, we develop a quantitative explanation of the electrical activity observed in pancreatic islets. The proposed mechanism involves the voltage-dependent inward calcium and outward potassium currents described by Rorsman and Trube (1986), which are voltage-activated when an increase in the cytoplasmic ATP/ADP ratio decreases the conductance of the ATP-sensitive potassium channels. It is proposed that modulation of the ATP/ADP ratio occurs through calcium inhibition of oxidative phosphorylation. In this picture the mitochondria serve as a transducer of metabolic activity whose sensitivity is modulated by cytosolic calcium. Solution of the differential equations that describe this mechanism gives rise to both bursting and continuous spiking electrical activity similar to that observed experimentally. While the mechanism for bursting in this model involves the ATP/ADP ratio, the feedback is still provided by calcium, as originally proposed by Chay and Keizer (1983) using a Ca2+-activated potassium conductance. A mixed-model, which includes both ATP-sensitive and Ca2+-activated potassium conductances, also reproduces the experimentally observed electrical activity and may correspond more closely to the actual situation in vivo.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkhammar P., Nilsson T., Rorsman P., Berggren P. O. Inhibition of ATP-regulated K+ channels precedes depolarization-induced increase in cytoplasmic free Ca2+ concentration in pancreatic beta-cells. J Biol Chem. 1987 Apr 25;262(12):5448–5454. [PubMed] [Google Scholar]
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Harrison D. E., Ashcroft S. J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. 1984 Nov 29-Dec 5Nature. 312(5993):446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Atwater I., Dawson C. M., Ribalet B., Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J Physiol. 1979 Mar;288:575–588. [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Dawson C. M., Scott A., Eddlestone G., Rojas E. The nature of the oscillatory behaviour in electrical activity from pancreatic beta-cell. Horm Metab Res Suppl. 1980;Suppl 10:100–107. [PubMed] [Google Scholar]
- Chay T. R., Keizer J. Minimal model for membrane oscillations in the pancreatic beta-cell. Biophys J. 1983 May;42(2):181–190. doi: 10.1016/S0006-3495(83)84384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chay T. R. On the effect of the intracellular calcium-sensitive K+ channel in the bursting pancreatic beta-cell. Biophys J. 1986 Nov;50(5):765–777. doi: 10.1016/S0006-3495(86)83517-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chay T. R. The effect of inactivation of calcium channels by intracellular Ca2+ ions in the bursting pancreatic beta-cells. Cell Biophys. 1987 Dec;11:77–90. doi: 10.1007/BF02797114. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Ikeuchi M., Fujimoto W. Y. Lowering of pHi inhibits Ca2+-activated K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):269–271. doi: 10.1038/311269a0. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Satin L. S., Ashford M. L., Hales C. N. ATP-sensitive K+ channels in pancreatic beta-cells. Spare-channel hypothesis. Diabetes. 1988 May;37(5):495–498. doi: 10.2337/diab.37.5.495. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Findlay I., Petersen O. H., Wollheim C. B. ATP-sensitive K+ channels in an insulin-secreting cell line are inhibited by D-glyceraldehyde and activated by membrane permeabilization. J Membr Biol. 1986;93(3):271–279. doi: 10.1007/BF01871181. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., West-Jordan J. A., Abraham R. J., Edwards R. H., Petersen O. H. The gating of nucleotide-sensitive K+ channels in insulin-secreting cells can be modulated by changes in the ratio ATP4-/ADP3- and by nonhydrolyzable derivatives of both ATP and ADP. J Membr Biol. 1988 Sep;104(2):165–177. doi: 10.1007/BF01870928. [DOI] [PubMed] [Google Scholar]
- Fein A., Tsacopoulos M. Activation of mitochondrial oxidative metabolism by calcium ions in Limulus ventral photoreceptor. Nature. 1988 Feb 4;331(6155):437–440. doi: 10.1038/331437a0. [DOI] [PubMed] [Google Scholar]
- Findlay I., Dunne M. J., Petersen O. H. High-conductance K+ channel in pancreatic islet cells can be activated and inactivated by internal calcium. J Membr Biol. 1985;83(1-2):169–175. doi: 10.1007/BF01868748. [DOI] [PubMed] [Google Scholar]
- Himmel D. M., Chay T. R. Theoretical studies on the electrical activity of pancreatic beta-cells as a function of glucose. Biophys J. 1987 Jan;51(1):89–107. doi: 10.1016/S0006-3495(87)83314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
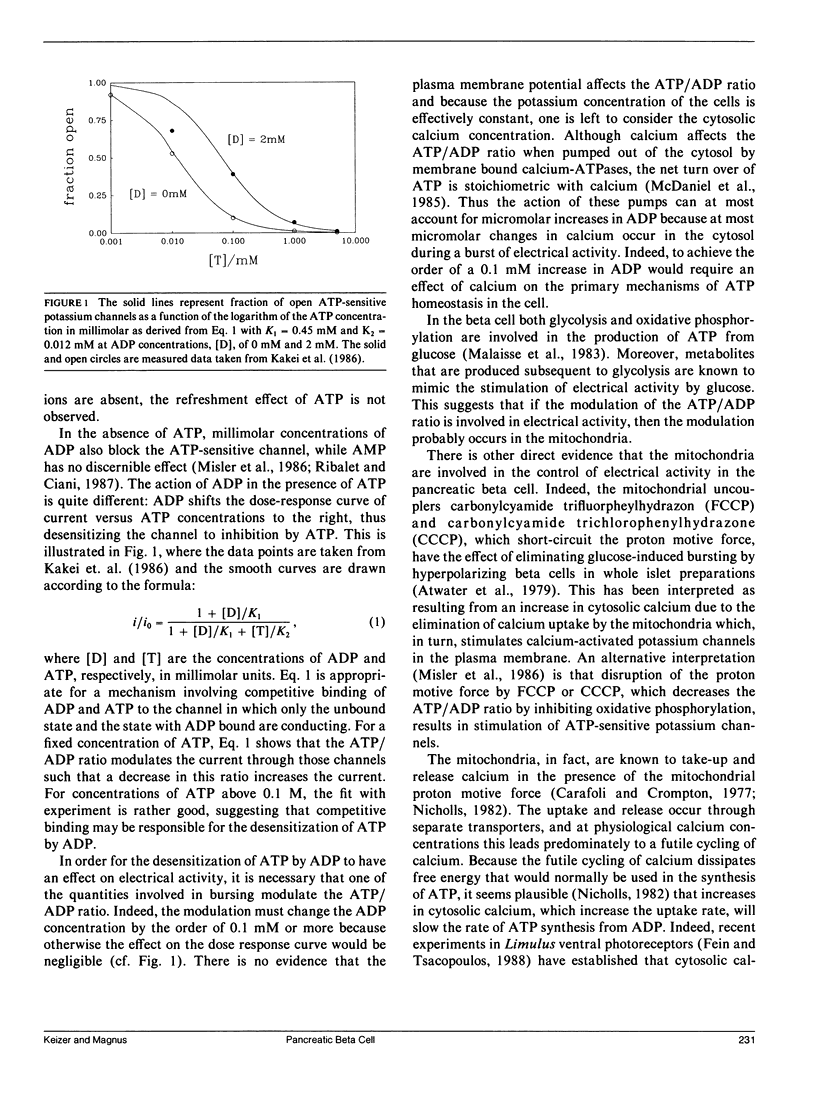
- Kakei M., Kelly R. P., Ashcroft S. J., Ashcroft F. M. The ATP-sensitivity of K+ channels in rat pancreatic B-cells is modulated by ADP. FEBS Lett. 1986 Nov 10;208(1):63–66. doi: 10.1016/0014-5793(86)81533-2. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Sener A. Anomeric specificity of hexose metabolism in pancreatic islets. Physiol Rev. 1983 Jul;63(3):773–786. doi: 10.1152/physrev.1983.63.3.773. [DOI] [PubMed] [Google Scholar]
- Meissner H. P., Atwater I. J. The kinetics of electrical activity of beta cells in response to a "square wave" stimulation with glucose or glibenclamide. Horm Metab Res. 1976 Jan;8(1):11–16. doi: 10.1055/s-0028-1093685. [DOI] [PubMed] [Google Scholar]
- Meissner H. P. Electrical characteristics of the beta-cells in pancreatic islets. J Physiol (Paris) 1976 Nov;72(6):757–767. [PubMed] [Google Scholar]
- Misler S., Falke L. C., Gillis K., McDaniel M. L. A metabolite-regulated potassium channel in rat pancreatic B cells. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7119–7123. doi: 10.1073/pnas.83.18.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T., Zünkler B. J., Trube G. Dual effects of ATP on K+ currents of mouse pancreatic beta-cells. Pflugers Arch. 1987 Feb;408(2):133–138. doi: 10.1007/BF00581342. [DOI] [PubMed] [Google Scholar]
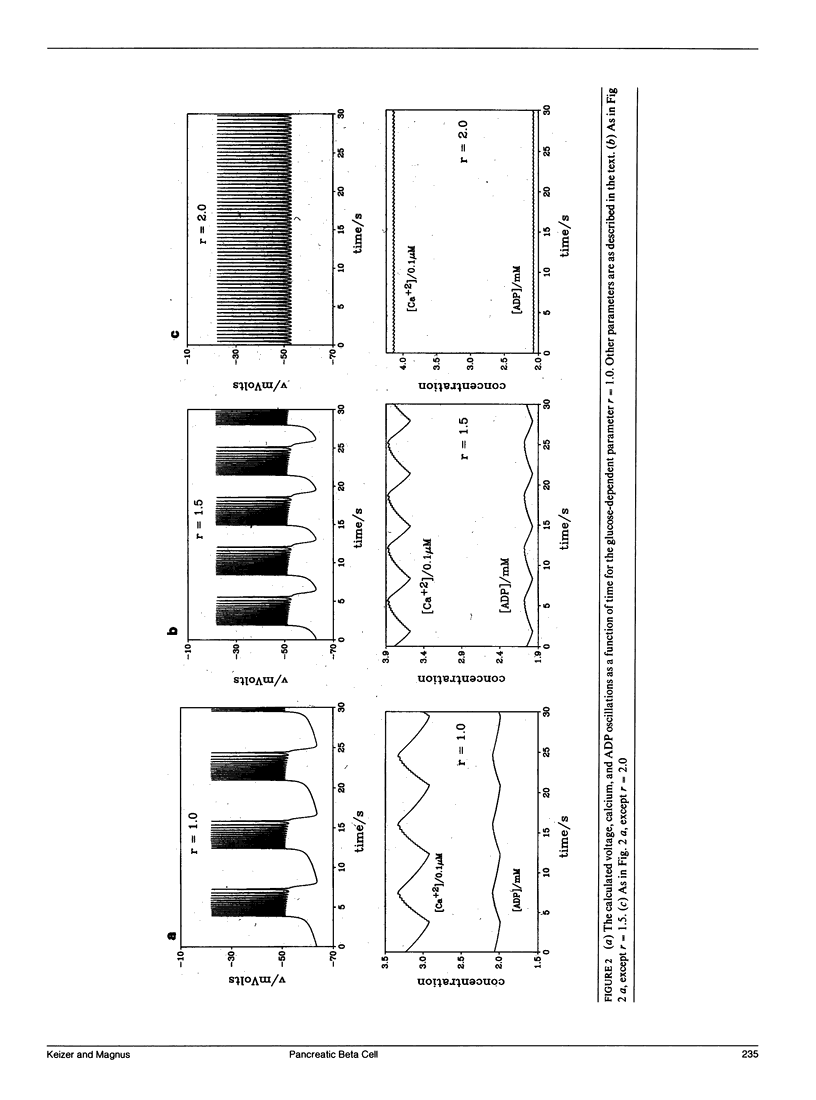
- Plant R. E. The effects of calcium++ on bursting neurons. A modeling study. Biophys J. 1978 Mar;21(3):217–237. doi: 10.1016/S0006-3495(78)85521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribalet B., Ciani S. Regulation by cell metabolism and adenine nucleotides of a K channel in insulin-secreting B cells (RIN m5F). Proc Natl Acad Sci U S A. 1987 Mar;84(6):1721–1725. doi: 10.1073/pnas.84.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinzel J., Chay T. R., Himmel D., Atwater I. Prediction of the glucose-induced changes in membrane ionic permeability and cytosolic Ca2+ by mathematical modeling. Adv Exp Med Biol. 1986;211:247–263. doi: 10.1007/978-1-4684-5314-0_23. [DOI] [PubMed] [Google Scholar]
- Rinzel J., Lee Y. S. Dissection of a model for neuronal parabolic bursting. J Math Biol. 1987;25(6):653–675. doi: 10.1007/BF00275501. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Abrahamsson H., Gylfe E., Hellman B. Dual effects of glucose on the cytosolic Ca2+ activity of mouse pancreatic beta-cells. FEBS Lett. 1984 May 7;170(1):196–200. doi: 10.1016/0014-5793(84)81398-8. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J Physiol. 1986 May;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman A., Rinzel J., Keizer J. Emergence of organized bursting in clusters of pancreatic beta-cells by channel sharing. Biophys J. 1988 Sep;54(3):411–425. doi: 10.1016/S0006-3495(88)82975-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Sharp G. W. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]


