Abstract
Pathogenic bacteria exploit the presence of various host cell molecules in order to colonize new tissues. Fibronectin is involved in a wide range of cell functions in vivo, and staphylococci, streptococci, and gonococci have evolved mechanisms to utilize this glycoprotein to mediate host cell binding. We show that elementary bodies (EB) from two biovars of Chlamydia trachomatis recruit fibronectin to their surfaces upon lysis of the host cell. We also demonstrate that a heparan sulfate lyase-sensitive molecule on chlamydial EB is responsible for binding at least a portion of this fibronectin.
In order for microbial pathogens to colonize new locations within their hosts, they must be able to adhere to host cells and avoid recognition by the host's immune system. One strategy to achieve these ends involves the utilization of host cell extracellular-matrix proteins, such as fibronectin and heparan sulfate-containing glycosaminoglycans, to mediate adherence. Fibronectin is a large glycoprotein that consists of several domains, each with a unique binding specificity for other extracellular matrix proteins, heparan sulfate, and host cell surface proteins. This ubiquitous molecule, a component of the extracellular matrix, is found in plasma and connective tissues and is known to serve diverse functions in vivo, including those of cell motility and substrate binding (6). Several microbial pathogens utilize fibronectin for adhesion to host cells by expressing fibronectin-binding proteins on their surfaces (4, 7). Neisseria gonorrhoeae makes use of two molecules manufactured by host cells, heparan sulfate and fibronectin. The adhesin expressed on the bacterial surface, OpaA, binds to host-cell-derived heparan sulfate. Heparan sulfate, in turn, binds to fibronectin, which then binds to integrins on the host cell surface. van Putten et al. (11) showed that fibronectin is required not only for bacterial binding but also for entry into host cells. Thus, gonococci use fibronectin as a molecular bridge to facilitate adherence and to initiate interaction with the host cell.
Chlamydia spp. are responsible for a number of diseases in humans, including ocular infections, respiratory ailments, and sexually transmitted diseases. Chlamydia trachomatis is a leading cause of blindness worldwide and is the most commonly reported bacterial pathogen in the United States. Despite the pervasiveness of the infections caused by Chlamydia, much about Chlamydia virulence and pathogenesis remains unresolved. Of specific importance are the molecules on the surfaces of chlamydiae, especially those required for binding and entry into host cells. A number of molecules on the surfaces of the bacteria have been identified and associated with attachment and entry into host cells; these molecules include major outer membrane protein (9), glycosylated major outer member protein (10), exoglycolipids (8), and heparan sulfate-like molecules (13).
We previously used single-chain, variable-fragment (scFv) monoclonal antibodies to characterize and identify molecules associated with the surfaces of chlamydiae (5). One particular scFv antibody, 3H1-E10, bound to a high-molecular-weight protein associated with the surfaces of infectious chlamydial elementary bodies (EB) that appeared to be of host cell origin. Given that other pathogenic bacteria exploit host cell proteins in order to promote infection, it is possible that chlamydiae utilize a similar strategy. The presence of a host cell molecule on the surfaces of chlamydiae may be responsible for modulating host-pathogen interactions. The purpose of this study was to identify the host cell molecule recognized by scFv antibody 3H1-E10.
Organisms.
C. trachomatis serovars L2 (L2/434/Bu) and D (D/UW-3/Cx) were grown in HeLa229 or L929 cell monolayers in T-150 flasks in RPMI medium containing 10% fetal bovine serum and vancomycin. Chlamydial EB were purified with 30 and 30 to 44% discontinuous Renografin gradients (E.R. Squibb and Sons, Princeton, N.J.) as previously described (3).
Immunoblots.
Cell and chlamydial lysates were resolved on sodium dodecyl sulfate-polyacrylamide gels and transferred to nitrocellulose. Nitrocellulose sheets were incubated for 1 h at room temperature in a phosphate-buffered saline-Tween (PBS-T) solution containing 5% nonfat dried milk. Proteins were probed with polyclonal rabbit anti-fibronectin antibody (1:10,000; Sigma, St. Louis, Mo.) for 1 h at room temperature and then washed three times in PBS-T. Immune reactions were detected with goat anti-rabbit antibody conjugated to horseradish peroxidase (HRP) (Sigma), diluted in PBS-T to 1:2,000, incubated for 1 h at room temperature, and washed three times in PBS-T before detection of immune reactions by chemiluminescence (ECL Kit; Amersham Pharmacia Biotech, Piscataway, N.J.).
Glycosaminoglycan-lyase treatment.
Fifty microliters of purified EB (1.1 × 108 inclusion-forming units/ml) was centrifuged for 5 min at 10,800 × g. Supernatants were aspirated, and the pellet from each tube was suspended in one of three solutions: 50 μl of PBS, 50 μl of PBS containing 0.05 U of heparitinase, or 50 μl of PBS containing 0.05 U of chondroitinase (Seikagaku America, Falmouth, Mass.). Suspensions were incubated for 90 min at 37°C and then centrifuged at 10,800 × g for 5 min. Supernatants were removed and saved, and the EB in the pellet fraction were washed twice with 50 μl of cold PBS and suspended to a final volume of 50 μl. Samples (12.5 μl) from each supernatant and pellet fraction were electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gels and transferred to nitrocellulose. Polyclonal rabbit anti-fibronectin antibody was used to probe for proteins on the nitrocellulose sheets, and immune reactions were detected by chemiluminescence as described above.
Fibronectin-binding assays.
A dot blot assay was used to test binding of fibronectin to chlamydial EB. Purified EB were divided into 150-μl aliquots (5 μg/ml). Serial twofold dilutions of fibronectin (Calbiochem, La Jolla, Calif.) were added to the aliquots of EB and incubated on ice for 30 min. The aliquots of EB were centrifuged for 2 min at 10,800 × g and washed three times in PBS-T. The final pellets were suspended in 150 μl of PBS-T, and 75 μl from each tube was added to each well in a dot blot manifold. All experiments were performed in duplicate. Dot blot wells were washed four times in PBS-T, incubated in blocking buffer (5% nonfat dried milk in PBS-T) for 1 h at room temperature, and washed three times in PBS-T. Primary antibody was added, and the wells were incubated for 1 h at room temperature. The wells were washed three times with 100 μl of PBS-T, and then the entire nitrocellulose sheet was washed three times in PBS-T. The nitrocellulose sheets were cut into strips and then incubated in enzyme-conjugated secondary antibody for 1 h at room temperature. Nitrocellulose strips were washed three times in PBS-T, and immune reactions were detected by chemiluminescence.
The high-molecular-weight protein in purified Chlamydia EB lysates is fibronectin.
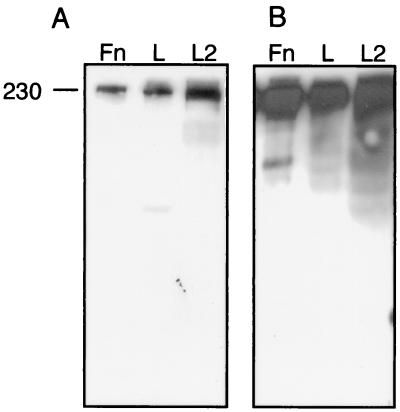
In the process of screening Chlamydia-specific scFv monoclonal antibodies by immunoblotting, one scFv antibody that binds a high-molecular-weight (∼230,000) protein in purified C. trachomatis serovar L2 EB and in uninfected L929 murine cell lysates was identified, namely, 3H1-E10 (5). The cellular localization pattern detected by scFv antibody 3H1-E10 in uninfected L929 cells as determined by immunofluorescence demonstrated that the antigen is on the surfaces of the cells and is a possible component of the extracellular matrix (5). Based on the size of the protein identified by immunoblotting and on the localization pattern from immunofluorescence, we hypothesized that the antigen is fibronectin. This hypothesis was tested by immunoblot analysis using lysates of L929 cells, purified C. trachomatis serovar L2 EB, and purified human fibronectin, which were probed with scFv antibody 3H1-E10. scFv antibody 3H1-E10 bound to a high-molecular-weight protein that comigrated in each sample (Fig. 1A), demonstrating that this antibody is immunoreactive to fibronectin. The specificity of scFv antibody 3H1-E10 for fibronectin was confirmed by performing a replicate immunoblot assay which showed that comigrating bands were reactive to a rabbit antiserum specific for fibronectin (Fig. 1B). We conclude that the Chlamydia-associated antigen recognized by 3H1-E10 is fibronectin.
FIG. 1.
Replicate immunoblots demonstrating the presence of fibronectin associated with purified chlamydial EB. Immunoblots probed with scFv antibody 3H1-E10 (A) and fibronectin-specific rabbit serum (B) are shown. Lane Fn, purified fibronectin; lane L, lysate of uninfected murine L929 cells; lane L2, lysate of C. trachomatis L2 EB harvested from murine L929 cells. A molecular weight marker (in thousands) is shown on the left.
Host-cell-derived fibronectin associates with EB from different Chlamydia biovars.
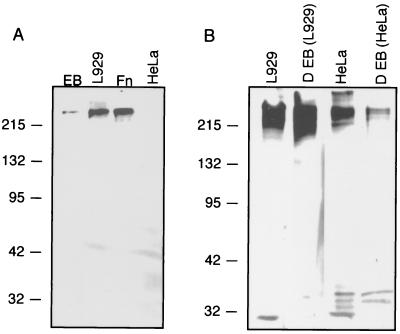
If fibronectin plays a role in the biology of C. trachomatis, it would be expected that this host cell protein would be recruited to the surfaces of the EB of different biovars. Immunoblot assays were performed with EB of serovars L2 and D, two biovars of C. trachomatis. Furthermore, HeLa cell lysates and EB harvested from HeLa cells were tested by the methods of Lindquist et al. (5), and a band immunoreactive to scFv antibody 3H1-E10 was not detected in HeLa cells, yet HeLa cells would be expected to contain fibronectin. As previously described by Lindquist et al. (5), scFv antibody 3H1-E10 was reactive to serovar L2 (and serovar D [data not shown]) EB purified from L929 cells but no reactivity was detected in HeLa cell lysates (Fig. 2A). However, when serovar L2 (data not shown) or serovar D EB lysates from organisms grown in HeLa cells and uninfected HeLa cell lysates were immunoblotted and probed with rabbit anti-fibronectin antibody, immunoreactive bands were detected (Fig. 2B). The immunoreactivities were much stronger in lysates of murine L929 cells than in HeLa cells, and concomitantly stronger reactivities were found in lysates of EB harvested from L929 cells than in those harvested from HeLa cells. These results indicate that the fibronectin associated with purified EB is of host cell origin and that the lack of detection of fibronectin in HeLa cells by scFv antibody 3H1-E10 results either from a quantifiably lower level of fibronectin associated with this cell line or from the production by HeLa cells of a modified form of fibronectin which is unrecognized by this scFv antibody. Fibronectin was associated with EB from two biovars (LGV and trachoma), suggesting that fibronectin binding is a conserved characteristic of C. trachomatis. While Kihlstrom et al. (2) showed that chlamydial EB bind exogenously added extracellular-matrix components, including fibronectin, our results demonstrate that fibronectin is already associated with purified EB, suggesting that this glycoprotein is recruited to the surfaces of the bacteria upon host cell lysis.
FIG. 2.
(A) scFv antibody 3H1-E10 is immunoreactive to fibronectin, as shown by immunoblot analysis, in lysates of murine L929 cells and C. trachomatis serovar L2. Lane EB, lysate of C. trachomatis L2 EB harvested from murine L929 cells; lane L929, lysate of uninfected murine L929 cells; lane Fn, purified fibronectin; lane HeLa, lysate of uninfected HeLa229 cells. Immunoblots were probed with scFv antibody 3H1-E10, and immunoreactive bands were detected with anti-E tag antibody and anti-mouse Fc-specific HRP-conjugated antibody. (B) Anti-fibronectin antibody binds C. trachomatis serovar D EB-associated antigen and an antigen in murine L929 cells and HeLa cells. Lane L929, lysate of uninfected murine L929 cells; lane D EB (L929), lysate of C. trachomatis serovar D EB harvested from murine L929 cells; lane HeLa, lysate of uninfected HeLa229 cells; lane D EB (HeLa), lysate of C. trachomatis serovar D EB harvested from HeLa229 cells. Immunoblots A and B contained equal amounts of host cell lysates and EB lysates. Blot B was probed with polyvalent rabbit serum specific for fibronectin; immunoreactive bands were detected with anti-rabbit specific HRP-conjugated antibody. Molecular weight markers (in thousands) are shown to the left of both panels.
The surfaces of Chlamydia EB have a receptors for fibronectin.
Although immunoblot assays of EB show that purified EB preparations contain host-cell-derived fibronectin, it is possible that this fibronectin represents a host cell contaminant that is merely carried over during purification and is not specifically interacting with EB. Thus, dot blot assays were used to test whether fibronectin binds to the surface of chlamydial EB. Purified EB were incubated with increasing amounts of fibronectin and washed with cold PBS before being probed with anti-fibronectin antibody (Fig. 3). This assay confirmed the existence of host cell fibronectin associating with the surfaces of EB. Furthermore, the dose-dependent binding demonstrated that purified EB are capable of binding exogenous fibronectin. The lack of binding by anti-Pgp3 antibody specific for a nonsurface EB protein demonstrated that the EB were intact. These results are consistent with earlier work showing that EB are able to bind several exogenous extracellular-matrix components, including collagen type I, heparan sulfate, fibronectin, vitronectin, and laminin (2).
FIG. 3.
Fibronectin binds to the surface of chlamydial EB, as shown by dot blotting. (Top) C. trachomatis serovar D EB were incubated with the indicated increasing amounts of exogenous human fibronectin. EB-bound fibronectin was detected with anti-fibronectin rabbit serum and a goat anti-rabbit HRP-conjugated antibody. (Bottom) Dot blots show (left to right) reactivities of EB to (i) monospecific anti-Pgp3 antibody, which is reactive to a highly expressed chlamydial cytosolic protein; (ii) the monoclonal antibody specific for OmpA, a highly expressed chlamydial surface protein; and (iii) the anti-Pgp3 antibody to EB, lysed prior to probing with antibody.
A heparan sulfate lyase-sensitive ligand mediates interaction between host-cell-derived fibronectin and Chlamydia EB.
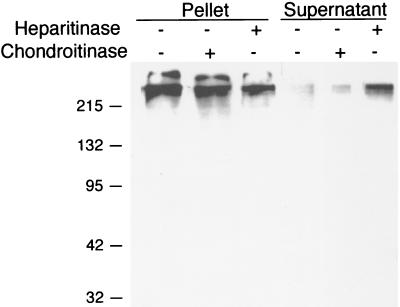
Fibronectin is a large protein involved in various aspects of the biology of multicellular organisms, including cell attachment, motility, and the initiation of intracellular signal transduction cascades. While many bacterial species employ fibronectin to mediate cell adherence, there are distinct differences in the microbial receptors for this host cell glycoprotein. Staphylococcus aureus (7) and Streptococcus pyogenes (4) have microbial surface components that recognize adhesive matrix molecules. In this case, fibronectin is bound directly by a bacterial surface protein. A second mechanism uses fibronectin as a molecular bridge; here, a bacterial surface protein binds to heparan sulfate, which then binds to fibronectin. This model has been described by van Putten et al. (11), who proposed that the cellular uptake of N. gonorrhoeae is dependent upon a protein-heparan-sulfate complex bridged to host cell integrins by fibronectin. On their surfaces, chlamydial EB display heparan sulfate-like components that are sensitive to heparan sulfate lyase (heparitinase) but are resistant to chondroitin sulfate lyase (13). Because fibronectin has heparan sulfate-binding domains, we tested whether EB-associated fibronectin was bound to EB-associated heparan sulfate. To experimentally address this question, EB were incubated in the presence of glycosaminoglycan lyases (chondroitinase or heparitinase) for 30 min and centrifuged in order to separate the soluble and insoluble fractions. The pellet was washed, and the pellet and supernatant protein fractions were then immunoblotted and probed with anti-fibronectin antibody (Fig. 4). The treatment of EB with chondroitinase had no effect, as fibronectin remained associated with the EB pellet fraction. In contrast, the treatment of EB with heparitinase resulted in the release of much of the EB-associated fibronectin into the supernatant fraction and in a concomitant reduction in the amount of fibronectin remaining in the EB pellet fraction. These results suggest that host cell fibronectin is binding to the heparan sulfate-like molecule on the surfaces of chlamydial EB.
FIG. 4.
Sensitivity of EB-associated fibronectin to heparitinase. C. trachomatis serovar D EB were incubated in the presence of heparitinase or chondroitinase for 30 min and washed with PBS. Fibronectin in the EB-containing pellet and the supernatant was detected by immunoblotting. The contents of all lanes were incubated with rabbit anti-fibronectin antibody and detected with goat anti-rabbit HRP conjugated antibody.
In the original studies by Zhang et al. (13), a heparitinase-sensitive heparan sulfate-like molecule that plays a role in cellular invasion was found on the surface of Chlamydia. We have shown that the EB-associated fibronectin is bound to EB by a heparitinase-sensitive molecule. The presence on the surface of Chlamydia of a heparan sulfate-like molecule that binds fibronectin is consistent with the methods used to select for the fibronectin-specific scFv antibody 3H1-E10. This monospecific antibody was eluted from EB by treating the bacteria with heparitinase (5). It has been shown that EB from both Chlamydia biovars display surface heparan sulfate, but there are significant differences between the biovars in the amount or structure of the heparan sulfate-like ligand (1). This is consistent with the finding that the two biovars apparently bound different amounts of fibronectin.
The finding of fibronectin associated with the surfaces of chlamydial EB raises questions as to the role of EB surface heparan sulfate in fibronectin binding and the role of fibronectin in mediating host cell adhesion or invasion. Zhang et al. (13) tested whether saturating EB with fibronectin would alter infectivity and found that it inhibited infectivity. Moreover, Wyrick et al. (12) tested whether integrins play a role in chlamydial infectivity using integrin-specific monoclonal antibodies and found that antibodies to α5- and β1-integrins did not affect chlamydial infectivity. Thus, whether the presence of host cell fibronectin on EB has biological significance in mediating host cell interactions such as adhesion, uptake, or the modulation of immune responses is intriguing but remains to be elucidated. The finding of host cell fibronectin bound to the surface of EB and to other uncharacterized proteins (5) suggests that bioactive components derived in host tissues could be expected to associate with EB in vivo and to modulate chlamydial pathogenesis. The determination of a specific role for fibronectin, heparan sulfate, or other chlamydial proteins in host cell adhesion and invasion will be unequivocally resolved only when the host cell receptor(s) is identified.
Acknowledgments
B.J.K. was supported by National Institutes of Health training grant GM07127, and the research was supported by National Institutes of Health grants AI42156 and AI32943.
Editor: D. L. Burns
REFERENCES
- 1.Chen, J. C., J. P. Zhang, and R. S. Stephens. 1996. Structural requirements of heparin binding to Chlamydia trachomatis. J. Biol. Chem. 271:11134-11140. [PubMed] [Google Scholar]
- 2.Kihlstrom, E., M. Majeed, B. Rozalska, and T. Wadstrom. 1992. Binding of Chlamydia trachomatis serovar L2 to collagen types I and IV, fibronectin, heparan sulphate, laminin and vitronectin. Zentralbl. Bakteriol. 277:329-333. [DOI] [PubMed] [Google Scholar]
- 3.Koehler, J. E., R. R. Burgess, N. E. Thompson, and R. S. Stephens. 1990. Chlamydia trachomatis RNA polymerase major σ subunit. Sequence and structural comparison of conserved and unique regions with Escherichia coli σ70 and Bacillus subtilis σ43. J. Biol. Chem. 265:13206-13214. [PubMed] [Google Scholar]
- 4.Kreikemeyer, B., S. R. Talay, and G. S. Chhatwal. 1995. Characterization of a novel fibronectin-binding surface protein in group A streptococci. Mol. Microbiol. 17:137-145. [DOI] [PubMed] [Google Scholar]
- 5.Lindquist, E. A., J. D. Marks, B. J. Kleba, and R. S. Stephens. 2002. Phage-display antibody detection of Chlamydia trachomatis-associated antigens. Microbiology 148:443-451. [DOI] [PubMed] [Google Scholar]
- 6.Romberger, D. J. 1997. Fibronectin. Int. J. Biochem. Cell Biol. 29:939-943. [DOI] [PubMed] [Google Scholar]
- 7.Signäs, C., G. Raucci, K. Jönsson, P. E. Lindgren, G. M. Anantharamaiah, M. Höök, and M. Lindberg. 1989. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc. Natl. Acad. Sci. USA 86:699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuart, E. S., S. M. Tirrell, and A. B. MacDonald. 1987. Characterization of an antigen secreted by Chlamydia-infected cell culture. Immunology 61:527-533. [PMC free article] [PubMed] [Google Scholar]
- 9.Su, H., L. Raymond, D. D. Rockey, E. Fischer, T. Hackstadt, and H. D. Caldwell. 1996. A recombinant Chlamydia trachomatis major outer membrane protein binds to heparan sulfate receptors on epithelial cells. Proc. Natl. Acad. Sci. USA 93:11143-11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson, A. F., and C. C. Kuo. 1994. Binding of the glycan of the major outer membrane protein of Chlamydia trachomatis to HeLa cells. Infect. Immun. 62:24-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Putten, J. P. M., T. D. Duensing, and R. L. Cole. 1998. Entry of OpaA+ gonococci into HEp-2 cells requires concerted action of glycosaminoglycans, fibronectin and integrin receptors. Mol. Microbiol. 29:369-379. [DOI] [PubMed] [Google Scholar]
- 12.Wyrick, P. B., C. H. Davis, and E. A. Wayner. 1994. Chlamydia trachomatis does not bind to alpha beta 1 integrins to colonize a human endometrial epithelial cell line cultured in vitro. Microb. Pathog. 17:159-166. [DOI] [PubMed] [Google Scholar]
- 13.Zhang, J. P., and R. S. Stephens. 1992. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell 69:861-869. [DOI] [PubMed] [Google Scholar]