Abstract
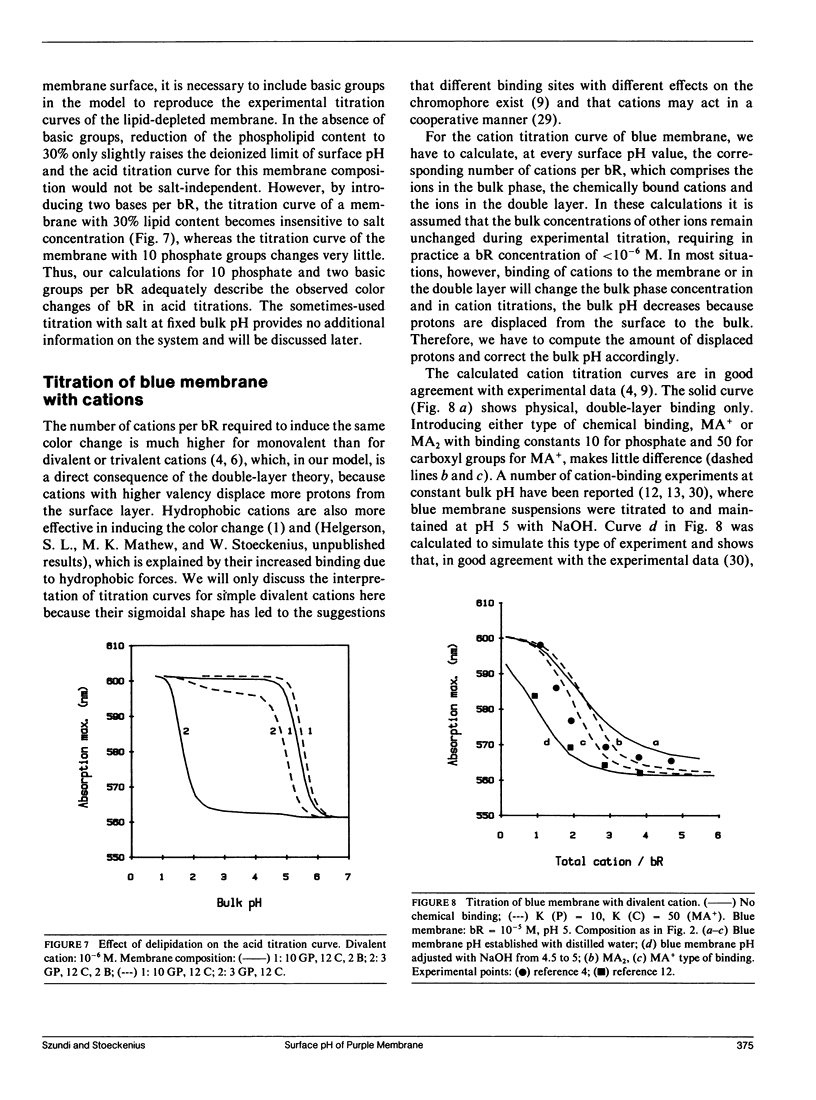
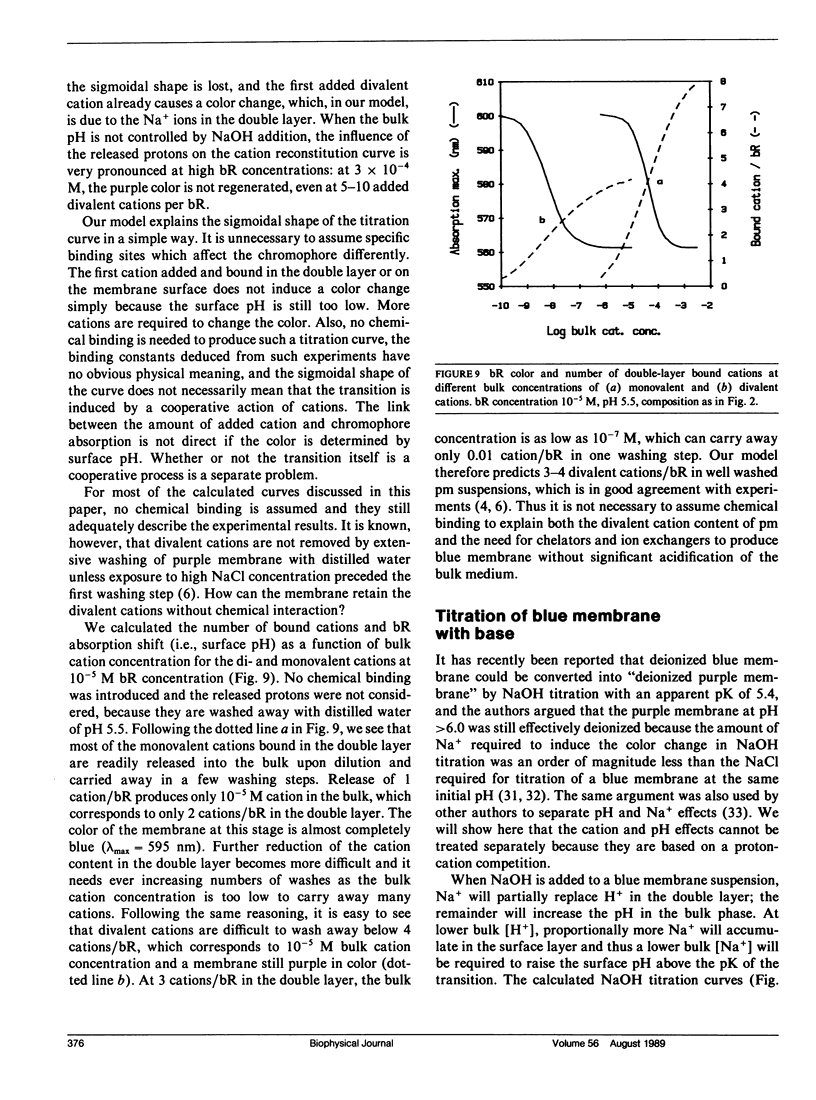
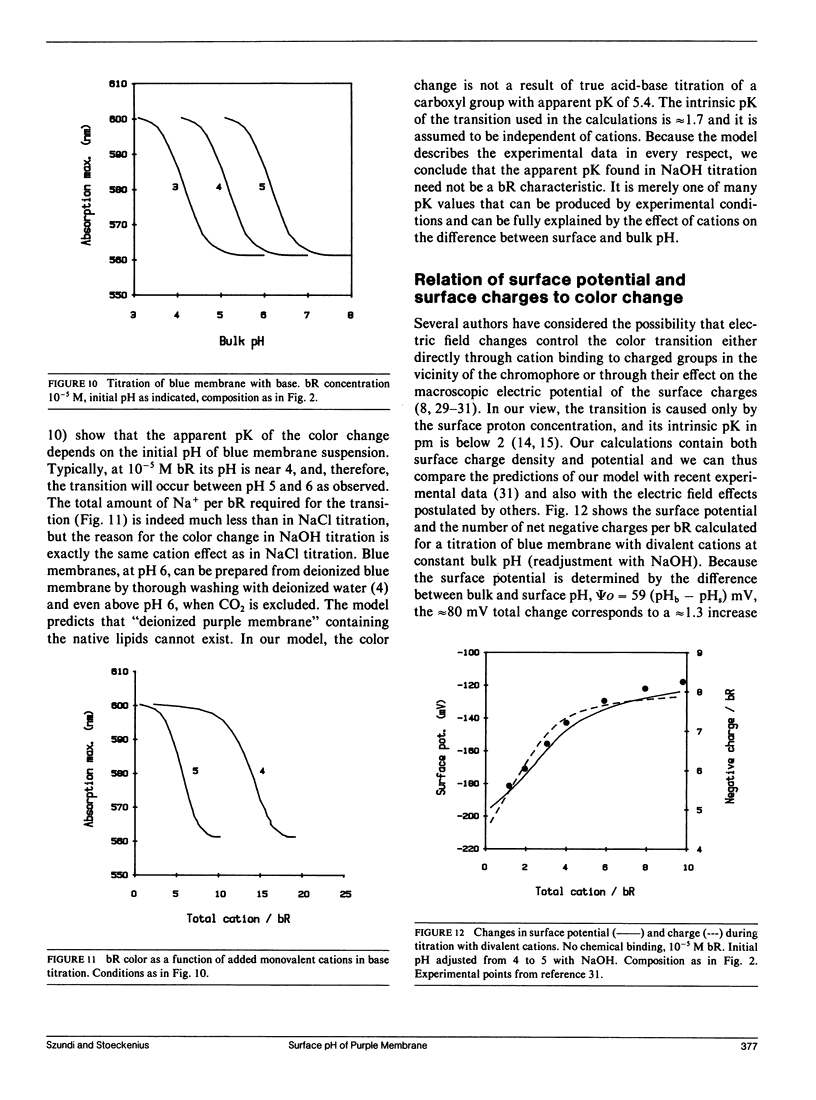
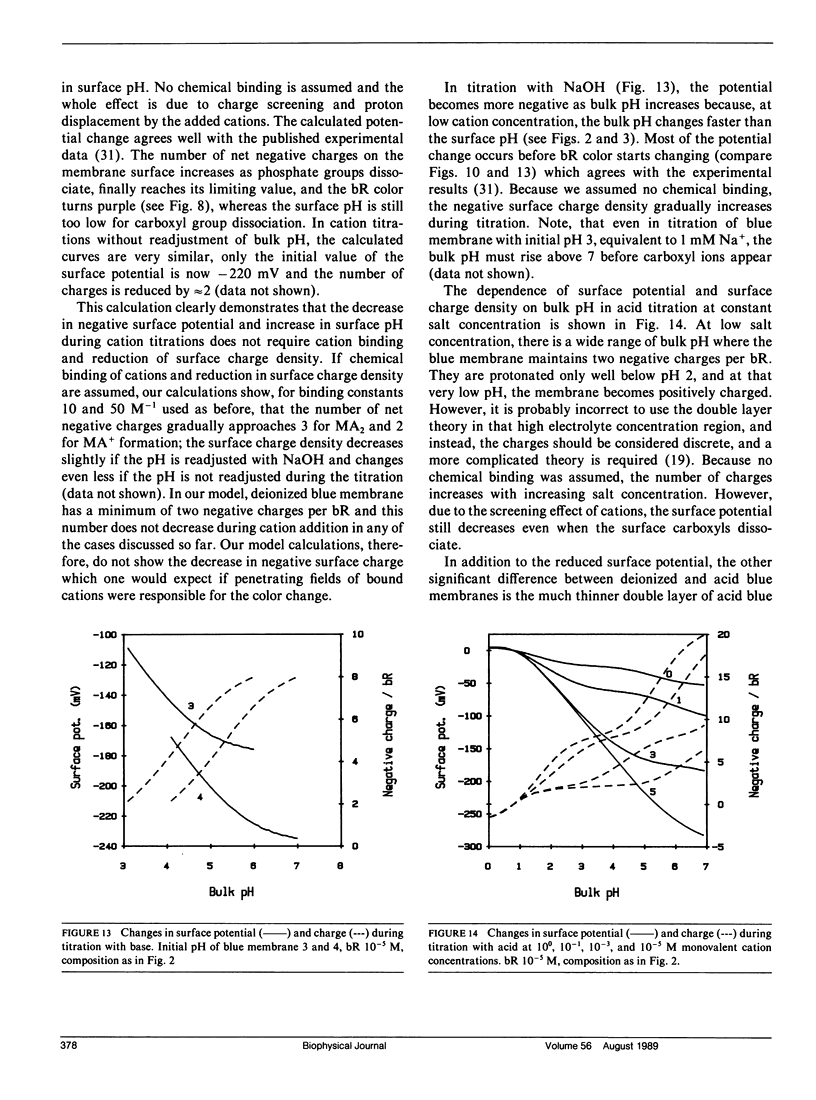
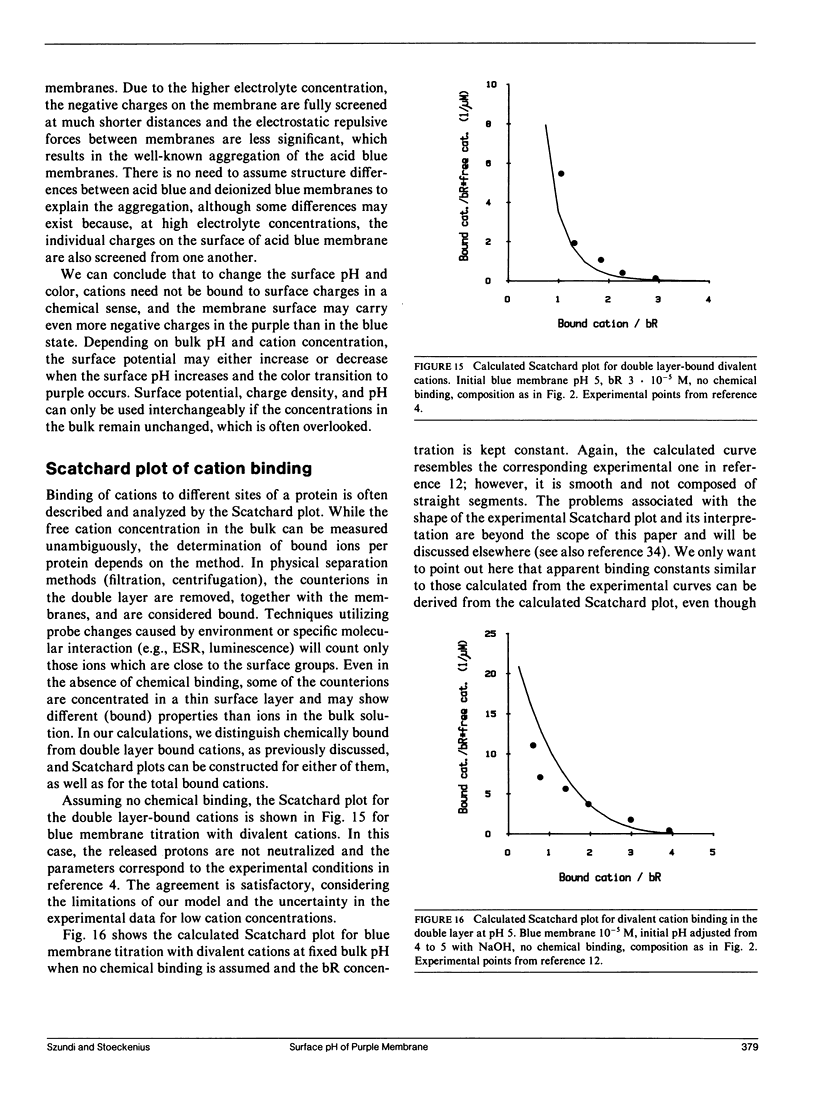
We have developed a surface model of purple membrane and applied it in an analysis of the purple-to-blue color change of bacteriorhodopsin which is induced by acidification or deionization. The model is based on dissociation and double layer theory and the known membrane structure. We calculated surface pH, ion concentrations, charge density, and potential as a function of bulk pH and concentration of mono- and divalent cations. At low salt concentrations, the surface pH is significantly lower than the bulk pH and it becomes independent of bulk pH in the deionized membrane suspension. Using an experimental acid titration curve for neutral, lipid-depleted membrane, we converted surface pH into absorption values. The calculated bacteriohodopsin color changes for acidification of purple, and titrations of deionized blue membrane with cations or base agree well with experimental results. No chemical binding is required to reproduce the experimental curves. Surface charge and potential changes in acid, base and cation titrations are calculated and their relation to the color change is discussed. Consistent with structural data, 10 primary phosphate and two basic surface groups per bacteriorhodopsin are sufficient to obtain good agreement between all calculated and experimental curves. The results provide a theoretical basis for our earlier conclusion that the purple-to-blue transition must be attributed to surface phenomena and not to cation binding at specific sites in the protein.
Full text
PDF


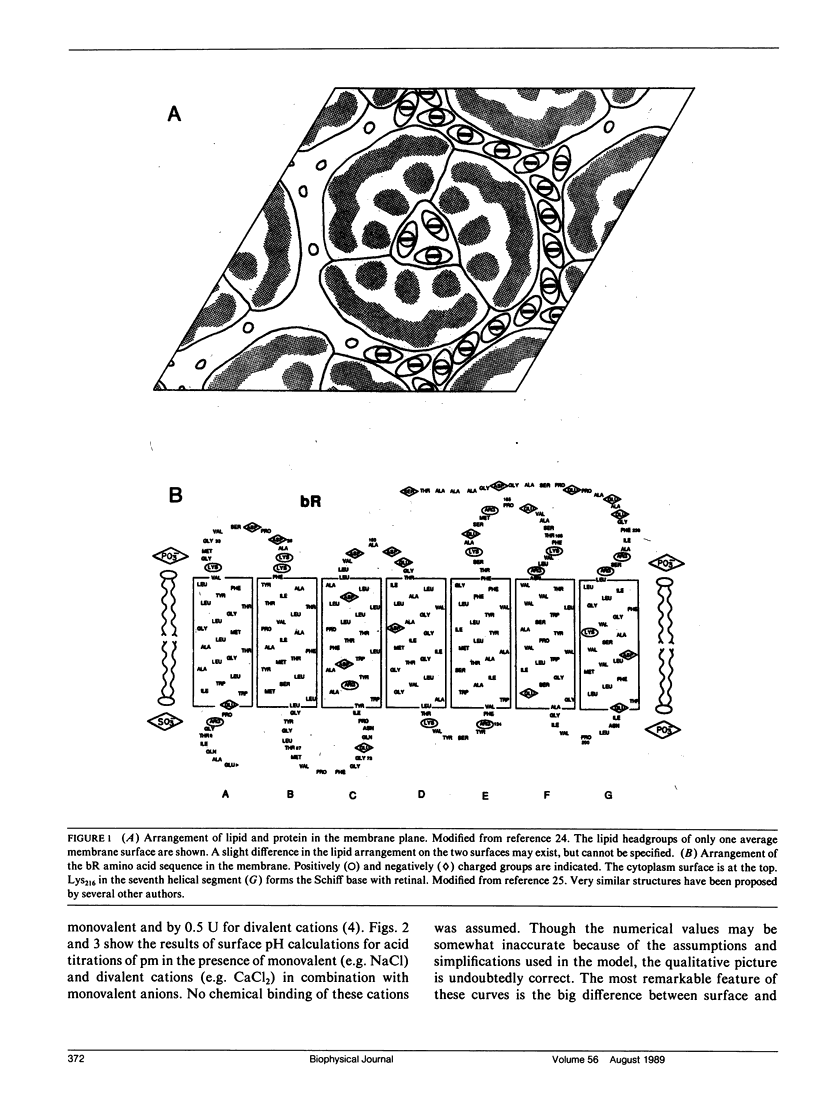
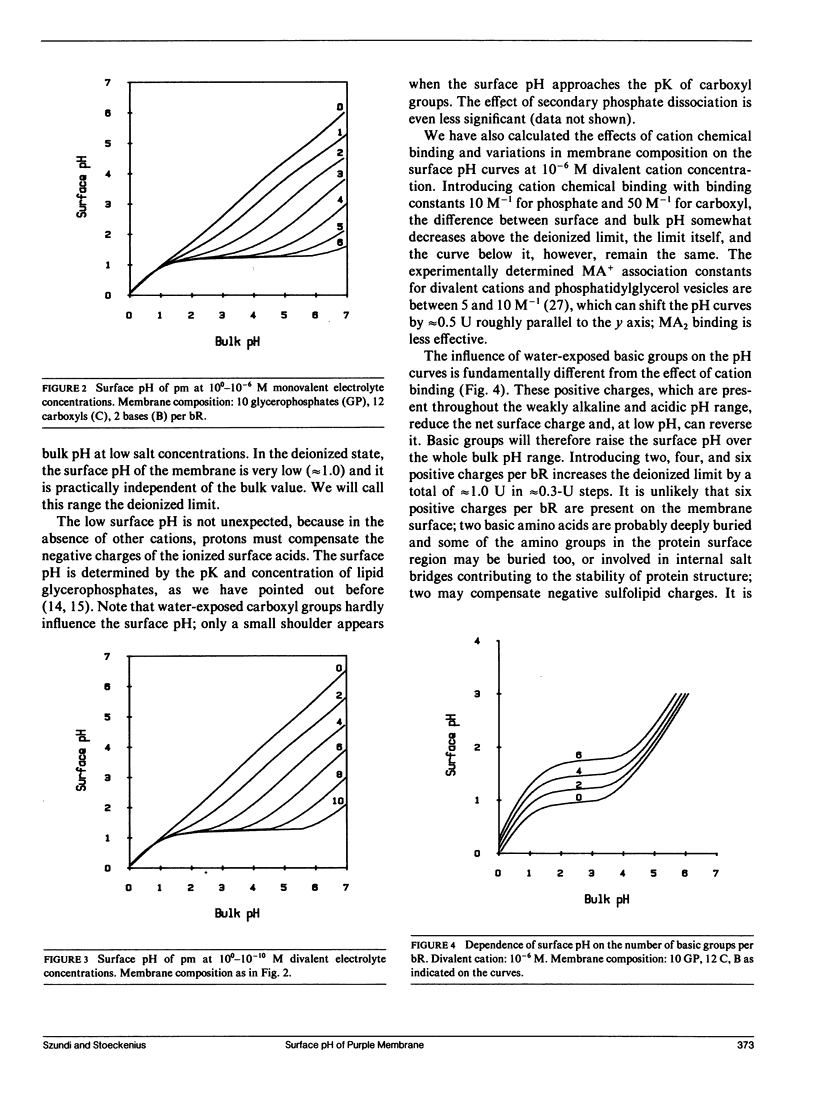
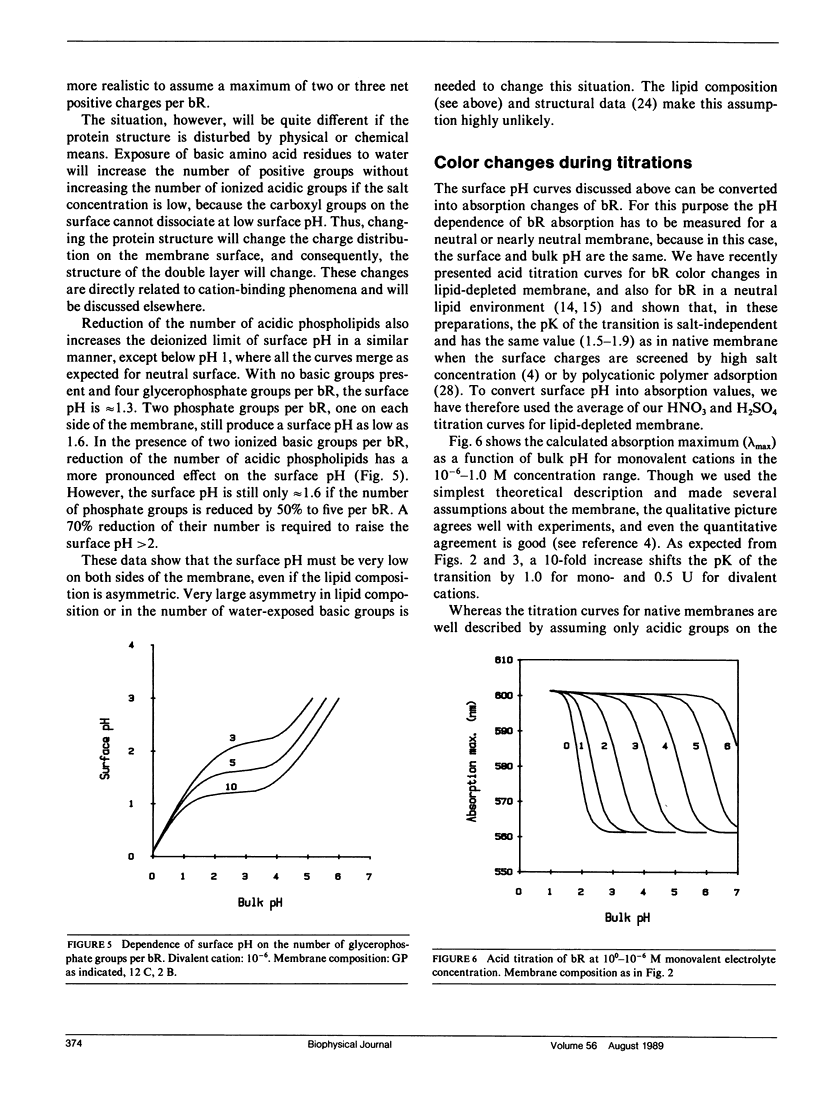




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amory D. E., Dufey J. E. Model for the electrolytic environment and electrostatic properties of biomembranes. J Bioenerg Biomembr. 1985 Jun;17(3):151–174. doi: 10.1007/BF00751059. [DOI] [PubMed] [Google Scholar]
- Ariki M., Lanyi J. K. Characterization of metal ion-binding sites in bacteriorhodopsin. J Biol Chem. 1986 Jun 25;261(18):8167–8174. [PubMed] [Google Scholar]
- Becher B., Cassim J. Y. Effects of bleaching and regeneration on the purple membrane structure of Halobaterium halobium. Biophys J. 1977 Sep;19(3):285–297. doi: 10.1016/s0006-3495(77)85588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaurock A. E., Stoeckenius W. Structure of the purple membrane. Nat New Biol. 1971 Sep 29;233(39):152–155. doi: 10.1038/newbio233152a0. [DOI] [PubMed] [Google Scholar]
- Carmeli C., Quintanilha A. T., Packer L. Surface charge changes in purple membranes and the photoreaction cycle of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4707–4711. doi: 10.1073/pnas.77.8.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Chen J. G., Govindjee R., Ebrey T. Cation binding by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1985 Jan;82(2):396–400. doi: 10.1073/pnas.82.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Jonas R., Melchiore S., Govindjee R., Ebrey T. G. Mechanism and role of divalent cation binding of bacteriorhodopsin. Biophys J. 1986 Mar;49(3):731–739. doi: 10.1016/S0006-3495(86)83699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronister E. L., Corcoran T. C., Song L., El-Sayed M. A. On the molecular mechanisms of the Schiff base deprotonation during the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8580–8584. doi: 10.1073/pnas.83.22.8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran T. C., Ismail K. Z., El-Sayed M. A. Evidence for the involvement of more than one metal cation in the Schiff base deprotonation process during the photocycle of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4094–4098. doi: 10.1073/pnas.84.12.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckmann S., Ottolenghi M., Korenstein R. Time-resolved absorbance changes induced by fast acidification of bacteriorhodopsin in vesicle systems. Biophys J. 1985 Jan;47(1):115–118. doi: 10.1016/S0006-3495(85)83883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duñach M., Padrós E., Seigneuret M., Rigaud J. L. On the molecular mechanism of the blue to purple transition of bacteriorhodopsin. UV-difference spectroscopy and electron spin resonance studies. J Biol Chem. 1988 Jun 5;263(16):7555–7559. [PubMed] [Google Scholar]
- Duñach M., Seigneuret M., Rigaud J. L., Padrós E. Influence of cations on the blue to purple transition of bacteriorhodopsin. Comparison of Ca2+ and Hg2+ binding and their effect on the surface potential. J Biol Chem. 1988 Nov 25;263(33):17378–17384. [PubMed] [Google Scholar]
- Duñach M., Seigneuret M., Rigaud J. L., Padrós E. The relationship between the chromophore moiety and the cation binding sites in bacteriorhodopsin. Biosci Rep. 1986 Nov;6(11):961–966. doi: 10.1007/BF01114972. [DOI] [PubMed] [Google Scholar]
- Fischer U., Oesterhelt D. Chromophore equilibria in bacteriorhodopsin. Biophys J. 1979 Nov;28(2):211–230. doi: 10.1016/S0006-3495(79)85172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K. A., Yanagimoto K., Stoeckenius W. Oriented adsorption of purple membrane to cationic surfaces. J Cell Biol. 1978 May;77(2):611–621. doi: 10.1083/jcb.77.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser R. M., Jubb J. S., Henderson R. Structural comparison of native and deoxycholate-treated purple membrane. Biophys J. 1985 Nov;48(5):775–780. doi: 10.1016/S0006-3495(85)83835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Jubb J. S., Whytock S. Specific labelling of the protein and lipid on the extracellular surface of purple membrane. J Mol Biol. 1978 Aug 5;123(2):259–274. doi: 10.1016/0022-2836(78)90325-x. [DOI] [PubMed] [Google Scholar]
- Herrmann T. R., Jayaweera A. R., Shamoo A. E. Interaction of europium(III) with phospholipid vesicles as monitored by laser-excited europium(III) luminescence. Biochemistry. 1986 Sep 23;25(19):5834–5838. doi: 10.1021/bi00367a074. [DOI] [PubMed] [Google Scholar]
- Jang D. J., el-Sayed M. A. Deprotonation of lipid-depleted bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5918–5922. doi: 10.1073/pnas.85.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katre N. V., Kimura Y., Stroud R. M. Cation binding sites on the projected structure of bacteriorhodopsin. Biophys J. 1986 Aug;50(2):277–284. doi: 10.1016/S0006-3495(86)83461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Ikegami A., Stoeckenius W. Salt and pH-dependent changes of the purple membrane absorption spectrum. Photochem Photobiol. 1984 Nov;40(5):641–646. doi: 10.1111/j.1751-1097.1984.tb05353.x. [DOI] [PubMed] [Google Scholar]
- Lau A., McLaughlin A., McLaughlin S. The adsorption of divalent cations to phosphatidylglycerol bilayer membranes. Biochim Biophys Acta. 1981 Jul 20;645(2):279–292. doi: 10.1016/0005-2736(81)90199-1. [DOI] [PubMed] [Google Scholar]
- Lind C., Höjeberg B., Khorana H. G. Reconstitution of delipidated bacteriorhodopsin with endogenous polar lipids. J Biol Chem. 1981 Aug 25;256(16):8298–8305. [PubMed] [Google Scholar]
- Marrero H., Rothschild K. J. Conformational changes in bacteriorhodopsin studied by infrared attenuated total reflection. Biophys J. 1987 Oct;52(4):629–635. doi: 10.1016/S0006-3495(87)83254-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S., Harary H. The hydrophobic adsorption of charged molecules to bilayer membranes: a test of the applicability of the stern equation. Biochemistry. 1976 May 4;15(9):1941–1948. doi: 10.1021/bi00654a023. [DOI] [PubMed] [Google Scholar]
- Moore T. A., Edgerton M. E., Parr G., Greenwood C., Perham R. N. Studies of an acid-induced species of purple membrane from Halobacterium halobium. Biochem J. 1978 May 1;171(2):469–476. doi: 10.1042/bj1710469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowery P. C., Lozier R. H., Chae Q., Tseng Y. W., Taylor M., Stoeckenius W. Effect of acid pH on the absorption spectra and photoreactions of bacteriorhodopsin. Biochemistry. 1979 Sep 18;18(19):4100–4107. doi: 10.1021/bi00586a007. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Packer L., Arrio B., Johannin G., Volfin P. Surface charge of purple membranes measured by laser Doppler velocimetry. Biochem Biophys Res Commun. 1984 Jul 18;122(1):252–258. doi: 10.1016/0006-291x(84)90467-4. [DOI] [PubMed] [Google Scholar]
- Renthal R., Cha C. H. Charge asymmetry of the purple membrane measured by uranyl quenching of dansyl fluorescence. Biophys J. 1984 May;45(5):1001–1006. doi: 10.1016/S0006-3495(84)84245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer P., Mathew M. K., Sperling W., Stoeckenius W. Retinal isomer ratio in dark-adapted purple membrane and bacteriorhodopsin monomers. Biochemistry. 1989 Jan 24;28(2):829–834. doi: 10.1021/bi00428a063. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Mathies R. A. Resonance Raman spectra of the acidified and deionized forms of bacteriorhodopsin. Biophys J. 1985 Feb;47(2 Pt 1):251–254. doi: 10.1016/s0006-3495(85)83899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szundi I., Stoeckenius W. Effect of lipid surface charges on the purple-to-blue transition of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3681–3684. doi: 10.1073/pnas.84.11.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szundi I., Stoeckenius W. Purple-to-blue transition of bacteriorhodopsin in a neutral lipid environment. Biophys J. 1988 Aug;54(2):227–232. doi: 10.1016/S0006-3495(88)82951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winiski A. P., McLaughlin A. C., McDaniel R. V., Eisenberg M., McLaughlin S. An experimental test of the discreteness-of-charge effect in positive and negative lipid bilayers. Biochemistry. 1986 Dec 16;25(25):8206–8214. doi: 10.1021/bi00373a013. [DOI] [PubMed] [Google Scholar]
- Zingsheim H. P., Neugebauer D. C., Henderson R. Properties of the two sides of the purple membrane correlated. J Mol Biol. 1978 Aug 5;123(2):275–278. doi: 10.1016/0022-2836(78)90326-1. [DOI] [PubMed] [Google Scholar]