Abstract
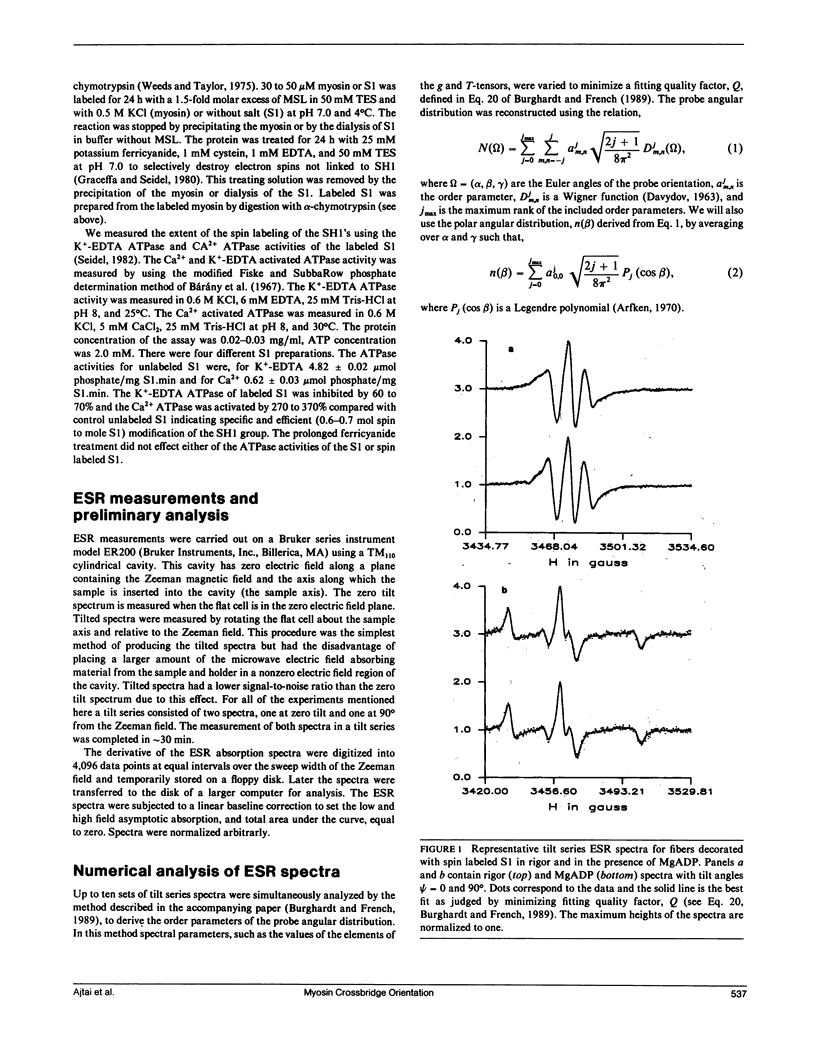
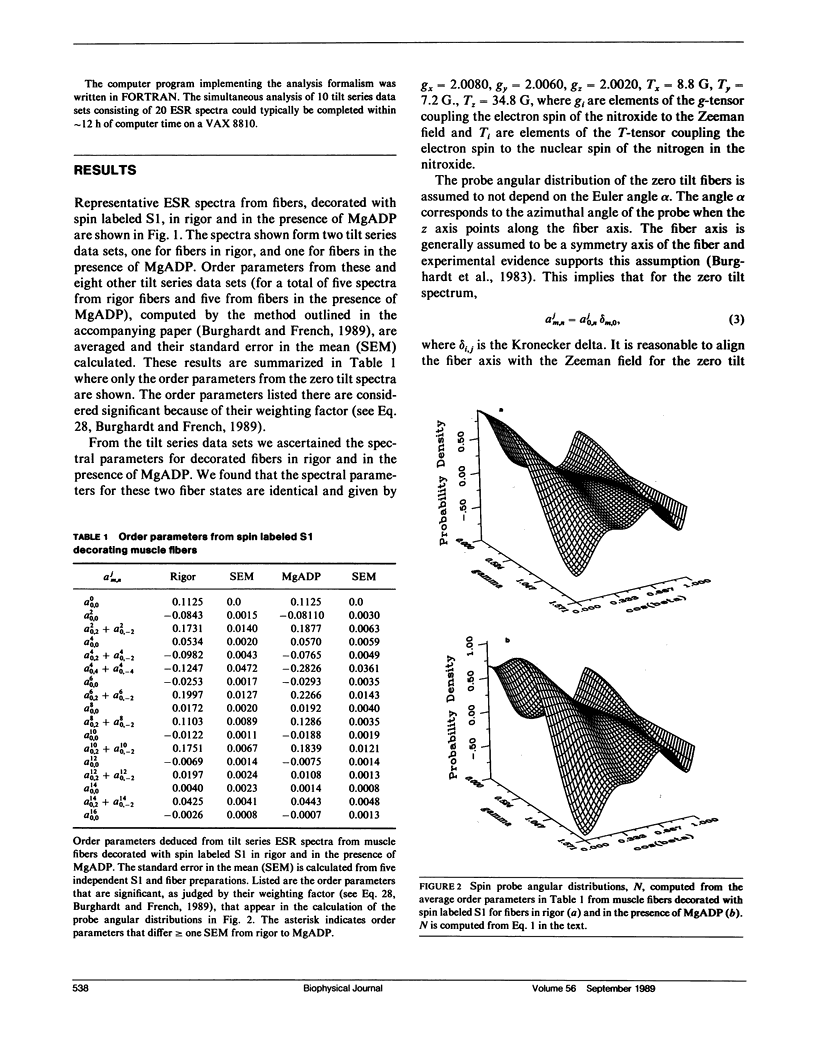
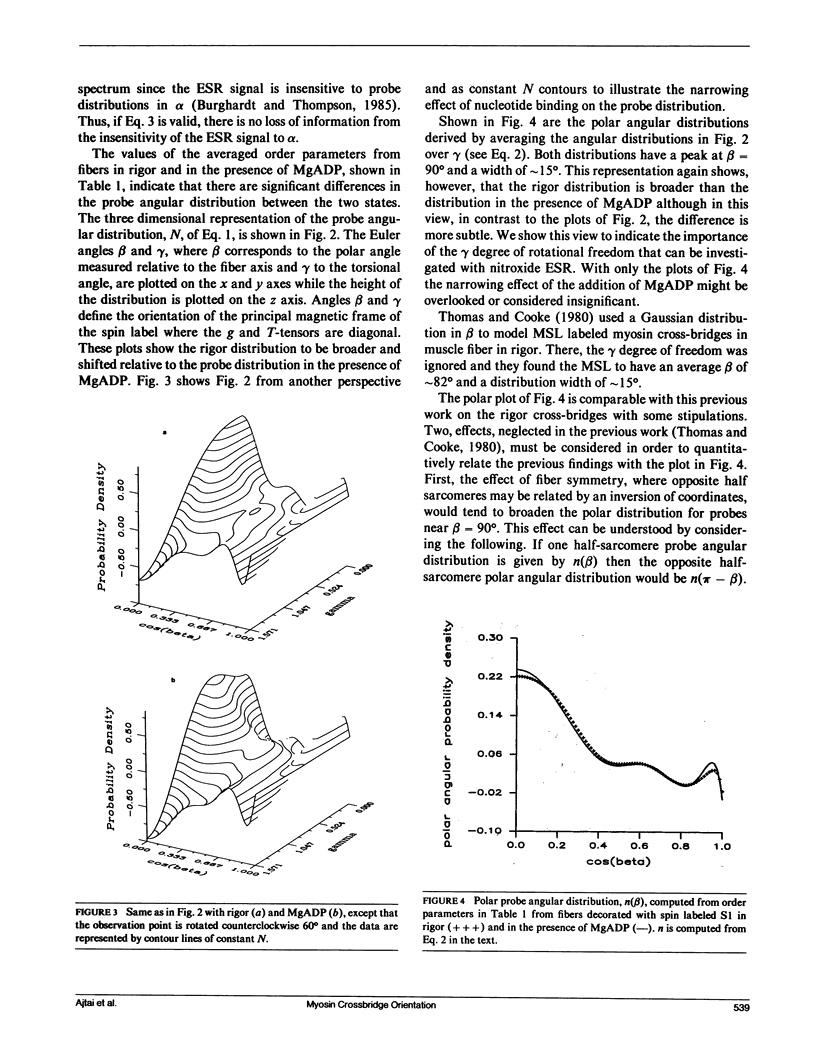
The tilt series electron spin resonance (ESR) spectrum from muscle fibers decorated with spin labeled myosin subfragment 1 (S1) was measured from fibers in rigor and in the presence of MgADP. ESR spectra were measured at low amplitude modulation of the static magnetic field to insure that a minimum of spectral lineshape distortion occurs. Ten tilt series ESR data sets were fitted simultaneously by the model-independent methodology described in the accompanying paper (Burghardt, T. P., and A. R. French, 1989. Biophys. J. 56:525-534). By this method the average and standard error in the mean of order parameters for the probe angular distribution were calculated for the two states of the fiber investigated. The average order parameters were used to reconstruct the probe angular distribution in two dimensions, one angular dimension corresponding to a polar angle measured relative to the fiber axis, and the other a torsional angular degree of freedom of the probe. We find that the probe angular distributions for the rigor and MgADP states of the fiber differ such that the rigor distribution is broader and shifted relative to the distribution in the presence of MgADP. The shape of the rigor distribution suggests the presence of two probe orientations, one similar to that in the presence of MgADP, and another at a different orientation. The shape of the distribution in the presence of MgADP suggests that the binding of the nucleotide to the rigor cross-bridge shifts the spin population into a more homogeneous one by causing a cross-bridge rotation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajtai K., Burghardt T. P. Fluorescent modification and orientation of myosin sulfhydryl 2 in skeletal muscle fibers. Biochemistry. 1989 Mar 7;28(5):2204–2210. doi: 10.1021/bi00431a035. [DOI] [PubMed] [Google Scholar]
- Ajtai K., Burghardt T. P. Observation of two orientations from rigor cross-bridges in glycerinated muscle fibers. Biochemistry. 1986 Oct 7;25(20):6203–6207. doi: 10.1021/bi00368a055. [DOI] [PubMed] [Google Scholar]
- Ajtai K., Burghardt T. P. Probe studies of the MgADP state of muscle cross-bridges: microscopic and wavelength-dependent fluorescence polarization from 1,5-IAEDANS-labeled myosin subfragment 1 decorating muscle fibers. Biochemistry. 1987 Jul 14;26(14):4517–4523. doi: 10.1021/bi00388a052. [DOI] [PubMed] [Google Scholar]
- Borejdo J., Assulin O., Ando T., Putnam S. Cross-bridge orientation in skeletal muscle measured by linear dichroism of an extrinsic chromophore. J Mol Biol. 1982 Jul 5;158(3):391–414. doi: 10.1016/0022-2836(82)90205-4. [DOI] [PubMed] [Google Scholar]
- Borejdo J., Putnam S., Morales M. F. Fluctuations in polarized fluorescence: evidence that muscle cross bridges rotate repetitively during contraction. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6346–6350. doi: 10.1073/pnas.76.12.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt T. P., Ajtai K. Fraction of myosin cross-bridges bound to actin in active muscle fibers: estimation by fluorescence anisotropy measurements. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8478–8482. doi: 10.1073/pnas.82.24.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt T. P., Ando T., Borejdo J. Evidence for cross-bridge order in contraction of glycerinated skeletal muscle. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7515–7519. doi: 10.1073/pnas.80.24.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt T. P., French A. R. Reconstruction of the probe angular distribution from a series of electron spin resonance spectra of tilted oriented samples. Biophys J. 1989 Sep;56(3):525–534. doi: 10.1016/S0006-3495(89)82699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt T. P., Thompson N. L. Model-independent electron spin resonance for measuring order of immobile components in a biological assembly. Biophys J. 1985 Sep;48(3):401–409. doi: 10.1016/S0006-3495(85)83796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M., Conover T. E., Schliselfeld L. H., Gaetjens E., Goffart M. Relation of properties of isolated myosin to those of intact muscles of the cat and sloth. Eur J Biochem. 1967 Sep;2(2):156–164. doi: 10.1111/j.1432-1033.1967.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Cooke R., Crowder M. S., Thomas D. D. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982 Dec 23;300(5894):776–778. doi: 10.1038/300776a0. [DOI] [PubMed] [Google Scholar]
- Cooke R. The mechanism of muscle contraction. CRC Crit Rev Biochem. 1986;21(1):53–118. doi: 10.3109/10409238609113609. [DOI] [PubMed] [Google Scholar]
- Graceffa P., Seidel J. C. A reaction involving protein sulfhydryl groups, a bound spin-label, and K3Fe(CN)6 as a probe of sulfhydryl proximity in myosin. Biochemistry. 1980 Jan 8;19(1):33–39. doi: 10.1021/bi00542a006. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Kress M. Crossbridge behaviour during muscle contraction. J Muscle Res Cell Motil. 1985 Apr;6(2):153–161. doi: 10.1007/BF00713057. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Seidel J. C. Electron paramagnetic resonance of contractile systems. Methods Enzymol. 1982;85(Pt B):594–624. doi: 10.1016/0076-6879(82)85054-4. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Ishiwata S., Seidel J. C., Gergely J. Submillisecond rotational dynamics of spin-labeled myosin heads in myofibrils. Biophys J. 1980 Dec;32(3):873–889. doi: 10.1016/S0006-3495(80)85023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonomura Y., Appel P., Morales M. On the molecular weight of myosin. II. Biochemistry. 1966 Feb;5(2):515–521. doi: 10.1021/bi00866a017. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]


