Abstract
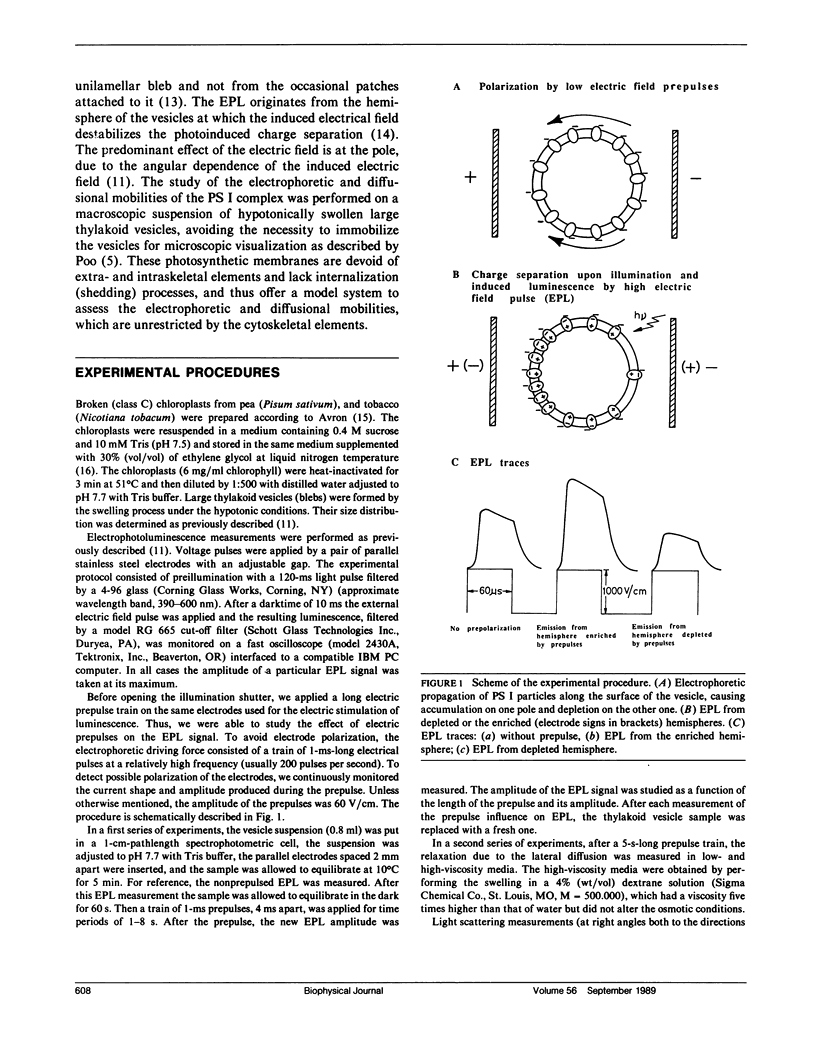
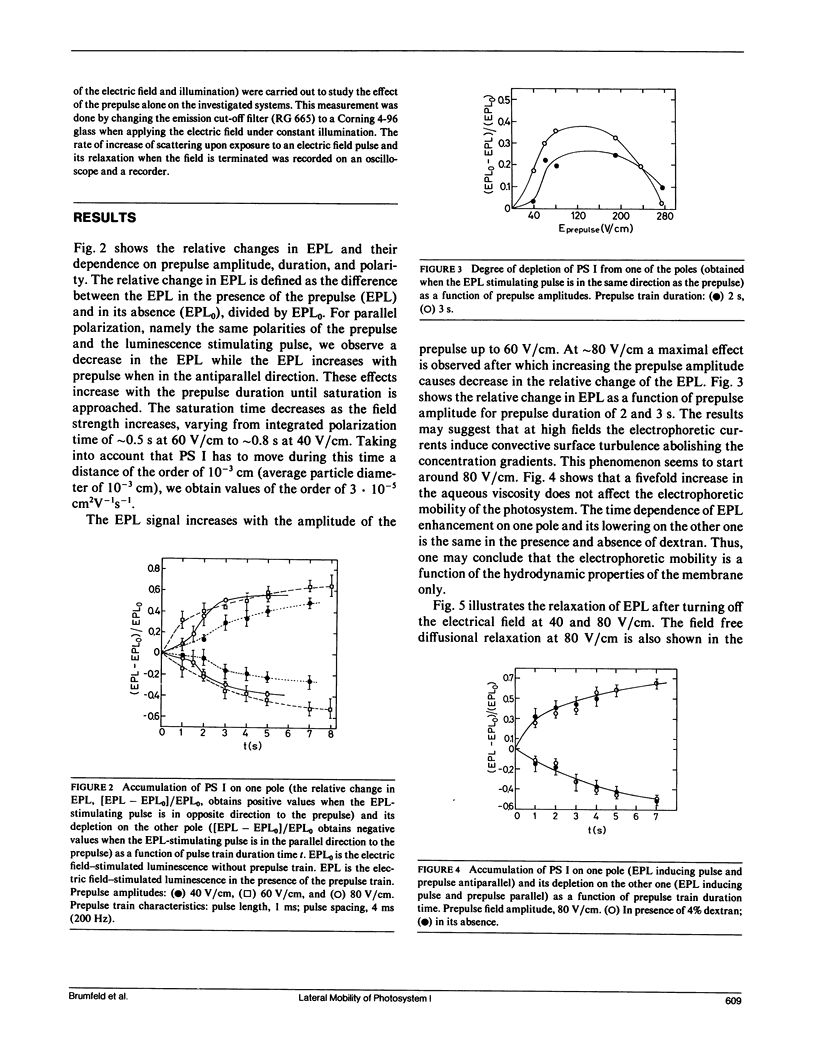
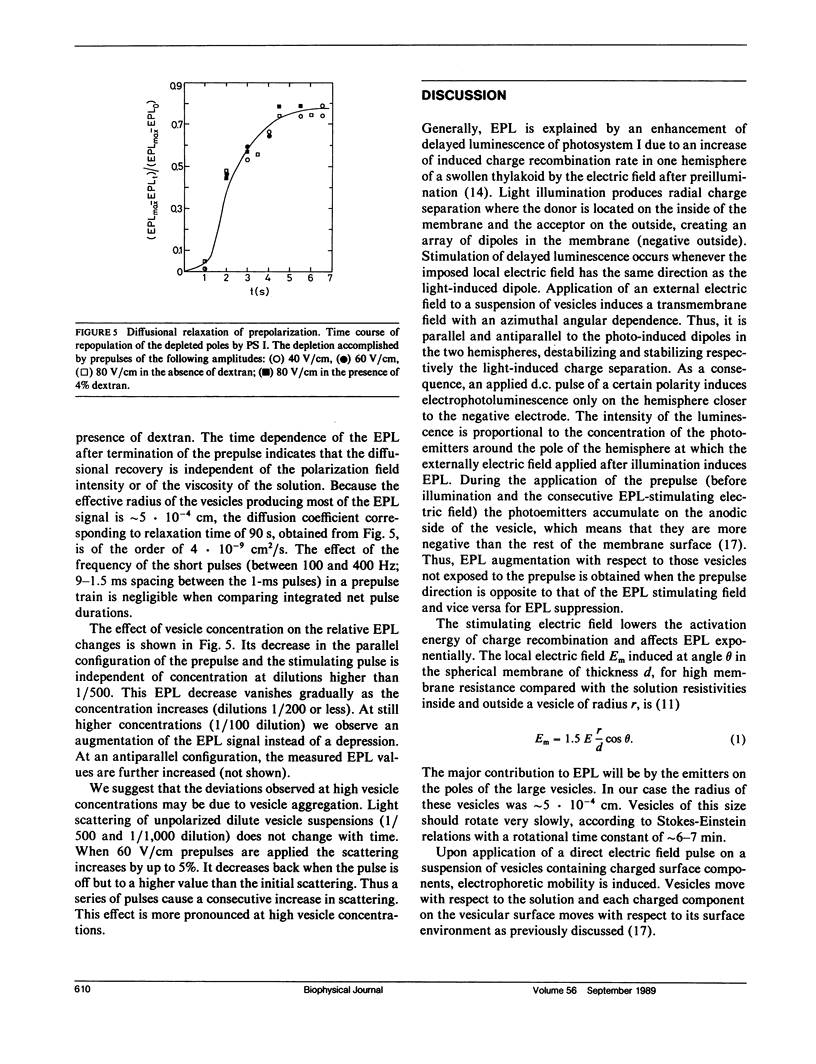
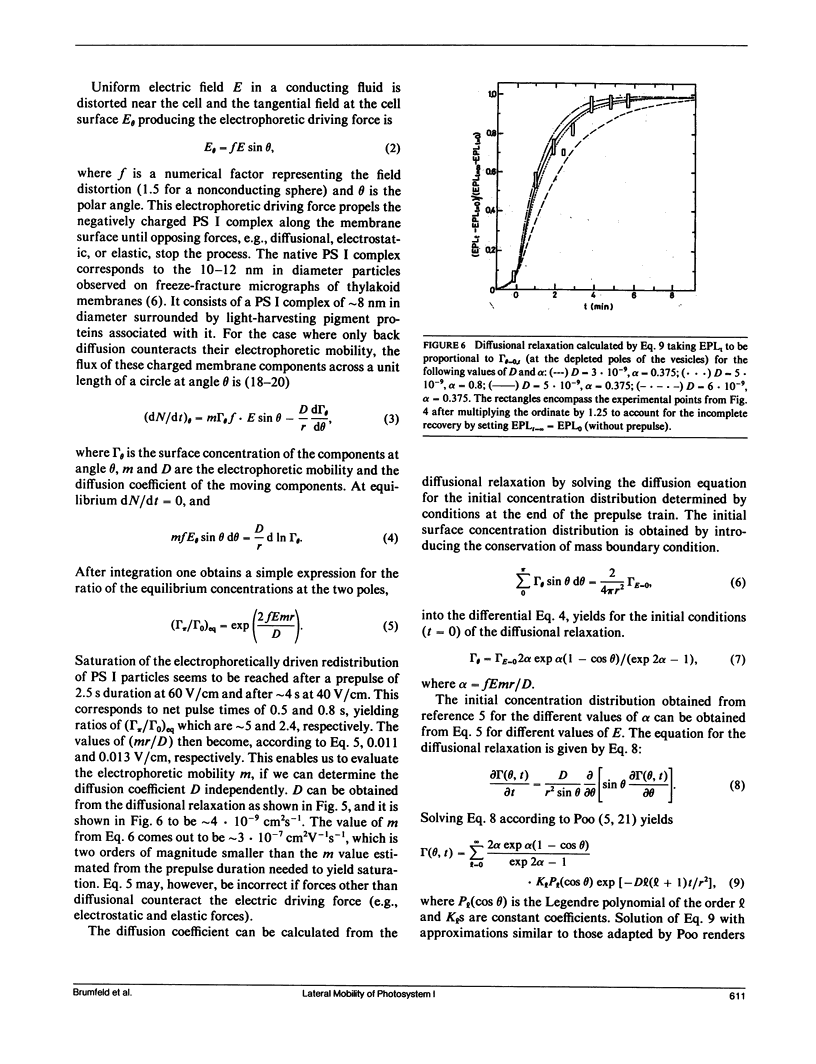
Electrophoretic movement of photosystem I (PS I) along the photosynthetic membrane of hypotonically swollen thylakoid vesicles was studied by analyzing the electric field-stimulated delayed luminescence (electrophotoluminescence) emitted from PS I. The electrophoretic mobility was inferred from the differences in electrophotoluminescence (EPL) of the photosynthetic vesicles in presence and absence of trains of low amplitude (<80 V/cm) prepulses of 1 ms duration at 4 ms spacing. The average apparent electric mobility, determined from the time course of EPL increase on one hemisphere or its decrease on the other one, as function of prepulse length and intensity was of the order of 3 · 10-5 cm2V-1s-1. The assymetric distribution of the PS I reached a steady state when the diffusional, electrostatic, and elastic forces balanced the electrophoretic driving force. A lateral diffusion coefficient of ∼5 · 10-9 cm2s-1 was found for the PS I complex from the diffusional relaxation after cessation of the electric field pulse train. Experimental conditions such as concentration, temperature, and viscosity of the aqueous solution were not critical for the effect. Between 23 and 150 electron charges per moving particle were estimated from the measured electrophoretic mobility.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Ellenson J. L., Sauer K. The electrophotoluminescence of chloroplasts. Photochem Photobiol. 1976 Feb;23(2):113–123. doi: 10.1111/j.1751-1097.1976.tb06782.x. [DOI] [PubMed] [Google Scholar]
- Farkas D. L., Korenstein R., Malkin S. Electrophotoluminescence and the electrical properties of the photosynthetic membrane. I. Initial kinetics and the charging capacitance of the membrane. Biophys J. 1984 Feb;45(2):363–373. doi: 10.1016/S0006-3495(84)84160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Ishihara A., Inman R. Lateral diffusion of proteins in membranes. Annu Rev Physiol. 1987;49:163–175. doi: 10.1146/annurev.ph.49.030187.001115. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F. Electrophoresis along cell membranes. Nature. 1977 Feb 17;265(5595):600–602. doi: 10.1038/265600a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin S., Poo M. M. The role of electro-osmosis in the electric-field-induced movement of charged macromolecules on the surfaces of cells. Biophys J. 1981 Apr;34(1):85–93. doi: 10.1016/S0006-3495(81)84838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E., Burke J. J., Arntzen C. J. Chlorophyll proteins of photosystem I. Plant Physiol. 1980 May;65(5):814–822. doi: 10.1104/pp.65.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M. In situ electrophoresis of membrane components. Annu Rev Biophys Bioeng. 1981;10:245–276. doi: 10.1146/annurev.bb.10.060181.001333. [DOI] [PubMed] [Google Scholar]
- Poo M., Lam J. W., Orida N., Chao A. W. Electrophoresis and diffusion in the plane of the cell membrane. Biophys J. 1979 Apr;26(1):1–21. doi: 10.1016/S0006-3495(79)85231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M., Robinson K. R. Electrophoresis of concanavalin A receptors along embryonic muscle cell membrane. Nature. 1977 Feb 17;265(5595):602–605. doi: 10.1038/265602a0. [DOI] [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A., Arntzen C. J. Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J Cell Biol. 1983 Nov;97(5 Pt 1):1327–1337. doi: 10.1083/jcb.97.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]


