Abstract
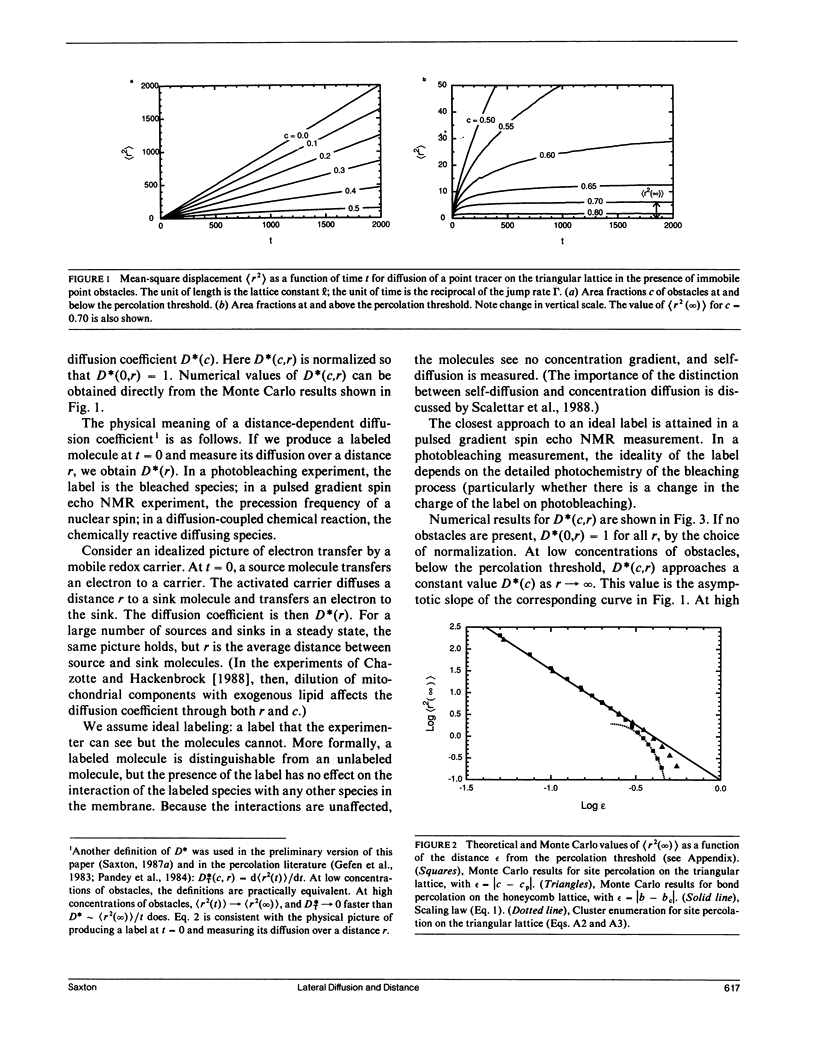
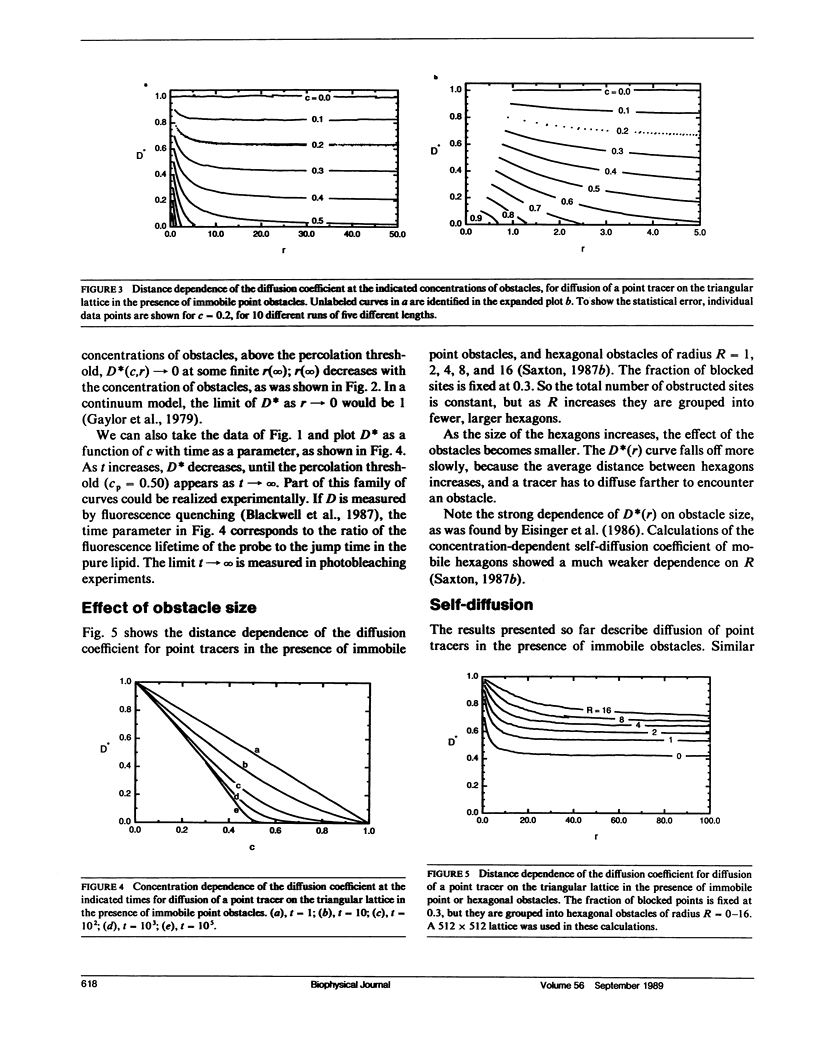
An understanding of the distance dependence of the lateral diffusion coefficient is useful in comparing the results of diffusion measurements made over different length scales, and in analyzing the kinetics of mobile redox carriers in organelles. A distance-dependent, concentration-dependent diffusion coefficient is defined, and it is evaluated by Monte Carlo calculations of a random walk by mobile point tracers in the presence of immobile obstacles on a triangular lattice, representing the diffusion of a lipid or a small protein in the presence of immobile membrane proteins. This work confirms and extends the milling crowd model of Eisinger, J., J. Flores, and W. P. Petersen (1986. Biophys J. 49:987-1001). Similar calculations for diffusion of mobile particles interacting by a hard-core repulsion yield the distance dependence of the self-diffusion coefficient. An expression for the range of short-range diffusion is obtained, and the distance scales for various diffusion measurements are summarized.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney J. R., Scalettar B. A., Owicki J. C. Self diffusion of interacting membrane proteins. Biophys J. 1989 May;55(5):817–833. doi: 10.1016/S0006-3495(89)82882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell M. F., Gounaris K., Zara S. J., Barber J. A method for estimating lateral diffusion coefficients in membranes from steady-state fluorescence quenching studies. Biophys J. 1987 May;51(5):735–744. doi: 10.1016/S0006-3495(87)83400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom J. A., Webb W. W. Lipid diffusibility in the intact erythrocyte membrane. Biophys J. 1983 Jun;42(3):295–305. doi: 10.1016/S0006-3495(83)84397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazotte B., Hackenbrock C. R. The multicollisional, obstructed, long-range diffusional nature of mitochondrial electron transport. J Biol Chem. 1988 Oct 5;263(28):14359–14367. [PubMed] [Google Scholar]
- Cowan A. E., Myles D. G., Koppel D. E. Lateral diffusion of the PH-20 protein on guinea pig sperm: evidence that barriers to diffusion maintain plasma membrane domains in mammalian sperm. J Cell Biol. 1987 Apr;104(4):917–923. doi: 10.1083/jcb.104.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoust J., Seigneuret M., Hervé P., Devaux P. F. Collisions between nitrogen-14 and nitrogen-15 spin-labels. 1. Lipid-lipid interactions in model membranes. Biochemistry. 1983 Jun 21;22(13):3137–3145. doi: 10.1021/bi00282a016. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Flores J., Petersen W. P. A milling crowd model for local and long-range obstructed lateral diffusion. Mobility of excimeric probes in the membrane of intact erythrocytes. Biophys J. 1986 May;49(5):987–1001. doi: 10.1016/S0006-3495(86)83727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger J., Scarlata S. F. The lateral fluidity of erythrocyte membranes. Temperature and pressure dependence. Biophys Chem. 1987 Dec;28(3):273–281. doi: 10.1016/0301-4622(87)80098-4. [DOI] [PubMed] [Google Scholar]
- Feix J. B., Yin J. J., Hyde J. S. Interactions of 14N:15N stearic acid spin-label pairs: effects of host lipid alkyl chain length and unsaturation. Biochemistry. 1987 Jun 30;26(13):3850–3855. doi: 10.1021/bi00387a017. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Gawrisch K., Stibenz D., Möps A., Arnold K., Linss W., Halbhuber K. J. The rate of lateral diffusion of phospholipids in erythrocyte microvesicles. Biochim Biophys Acta. 1986 Apr 25;856(3):443–447. doi: 10.1016/0005-2736(86)90135-5. [DOI] [PubMed] [Google Scholar]
- Golan D. E., Alecio M. R., Veatch W. R., Rando R. R. Lateral mobility of phospholipid and cholesterol in the human erythrocyte membrane: effects of protein-lipid interactions. Biochemistry. 1984 Jan 17;23(2):332–339. doi: 10.1021/bi00297a024. [DOI] [PubMed] [Google Scholar]
- Grasberger B., Minton A. P., DeLisi C., Metzger H. Interaction between proteins localized in membranes. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6258–6262. doi: 10.1073/pnas.83.17.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut J., Richter C., Cherry R. J., Winterhalter K. H., Kawato S. Rotation of cytochrome P-450. II. Specific interactions of cytochrome P-450 with NADPH-cytochrome P-450 reductase in phospholipid vesicles. J Biol Chem. 1982 Jun 25;257(12):7030–7036. [PubMed] [Google Scholar]
- Hackenbrock C. R., Chazotte B., Gupte S. S. The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J Bioenerg Biomembr. 1986 Oct;18(5):331–368. doi: 10.1007/BF00743010. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Ishihara A., Inman R. Lateral diffusion of proteins in membranes. Annu Rev Physiol. 1987;49:163–175. doi: 10.1146/annurev.ph.49.030187.001115. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P. A localized pattern photobleaching method for the concurrent analysis of rapid and slow diffusion processes. Biophys J. 1983 Aug;43(2):175–181. doi: 10.1016/S0006-3495(83)84338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo A. L., Wade C. G. Lipid lateral diffusion by pulsed nuclear magnetic resonance. Biochemistry. 1979 May 29;18(11):2300–2308. doi: 10.1021/bi00578a026. [DOI] [PubMed] [Google Scholar]
- Lenaz G., Fato R. Is ubiquinone diffusion rate-limiting for electron transfer? J Bioenerg Biomembr. 1986 Oct;18(5):369–401. doi: 10.1007/BF00743011. [DOI] [PubMed] [Google Scholar]
- Lenaz G. Role of mobility of redox components in the inner mitochondrial membrane. J Membr Biol. 1988 Sep;104(3):193–209. doi: 10.1007/BF01872322. [DOI] [PubMed] [Google Scholar]
- McCloskey M., Poo M. M. Protein diffusion in cell membranes: some biological implications. Int Rev Cytol. 1984;87:19–81. doi: 10.1016/s0074-7696(08)62439-0. [DOI] [PubMed] [Google Scholar]
- Quinn P., Griffiths G., Warren G. Density of newly synthesized plasma membrane proteins in intracellular membranes II. Biochemical studies. J Cell Biol. 1984 Jun;98(6):2142–2147. doi: 10.1083/jcb.98.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. The effect of mobile obstacles. Biophys J. 1987 Dec;52(6):989–997. doi: 10.1016/S0006-3495(87)83291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. The spectrin network as a barrier to lateral diffusion in erythrocytes. A percolation analysis. Biophys J. 1989 Jan;55(1):21–28. doi: 10.1016/S0006-3495(89)82776-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalettar B. A., Abney J. R., Owicki J. C. Theoretical comparison of the self diffusion and mutual diffusion of interacting membrane proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6726–6730. doi: 10.1073/pnas.85.18.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E. C. The mechanism of the conservation of energy of biological oxidations. Eur J Biochem. 1987 Aug 3;166(3):489–504. doi: 10.1111/j.1432-1033.1987.tb13542.x. [DOI] [PubMed] [Google Scholar]
- Smith L. M., McConnell H. M., Smith Baron A., Parce J. W. Pattern photobleaching of fluorescent lipid vesicles using polarized laser light. Biophys J. 1981 Jan;33(1):139–146. doi: 10.1016/S0006-3495(81)84877-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers A. E., Hackenbrock C. R. Rate of lateral diffusion of intramembrane particles: measurement by electrophoretic displacement and rerandomization. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6246–6250. doi: 10.1073/pnas.78.10.6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Träuble H., Sackmann E. Studies of the crystalline-liquid crystalline phase transition of lipid model membranes. 3. Structure of a steroid-lecithin system below and above the lipid-phase transition. J Am Chem Soc. 1972 Jun 28;94(13):4499–4510. doi: 10.1021/ja00768a015. [DOI] [PubMed] [Google Scholar]
- Yechiel E., Edidin M. Micrometer-scale domains in fibroblast plasma membranes. J Cell Biol. 1987 Aug;105(2):755–760. doi: 10.1083/jcb.105.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]


