Abstract
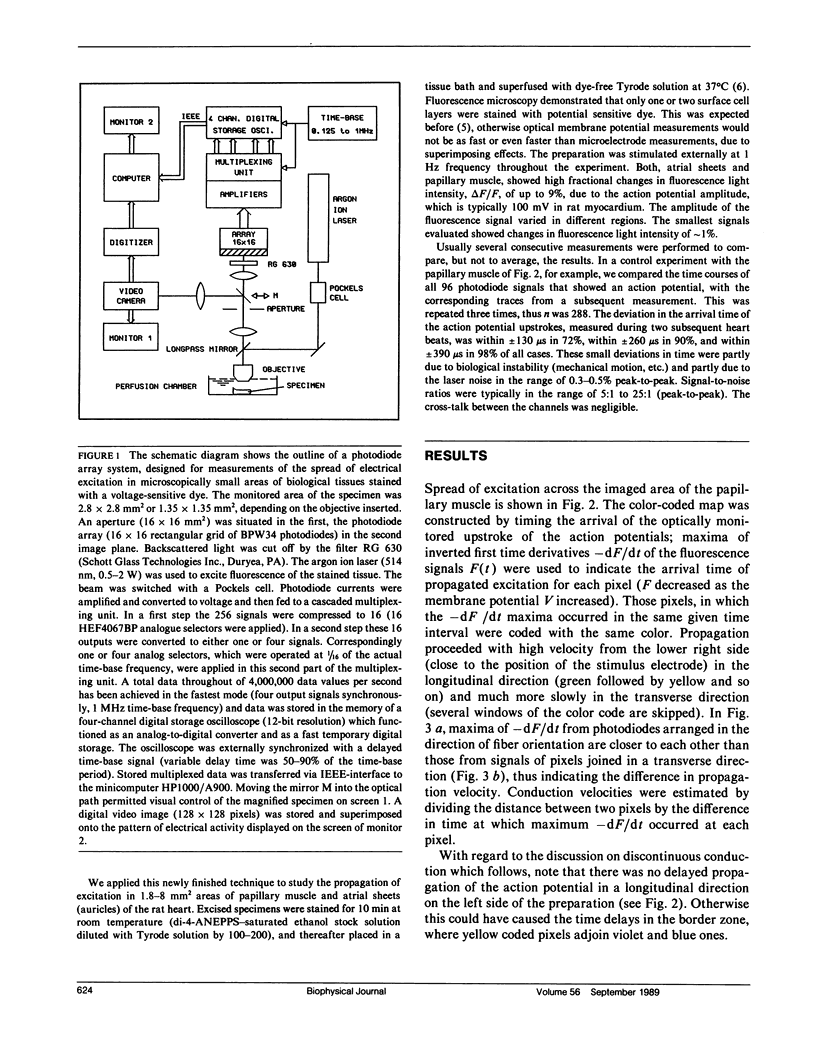
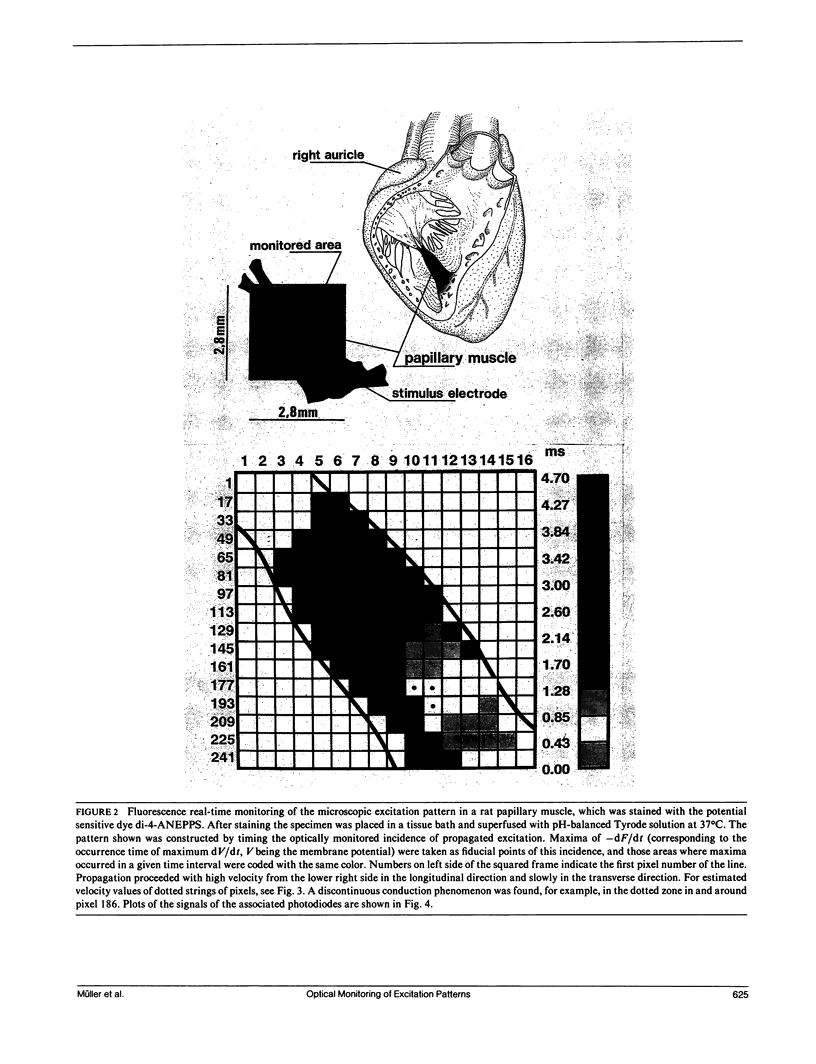
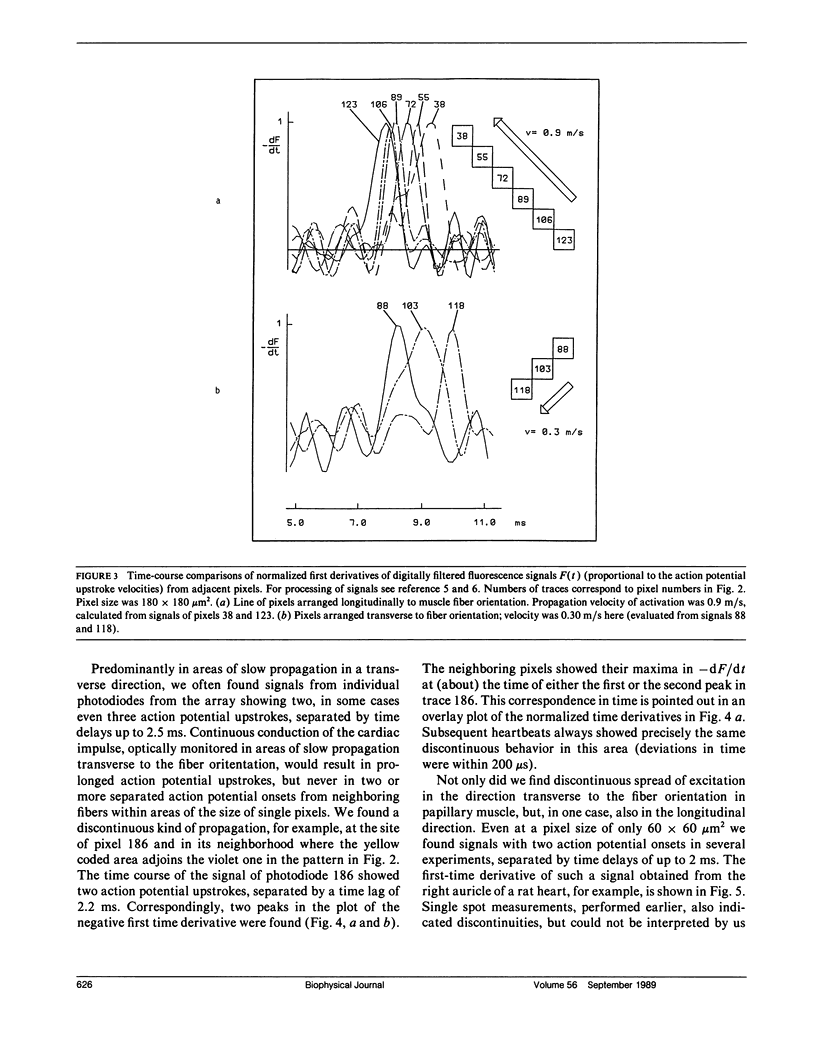
Many vital processes depend on the generation, changes, and conduction of cellular transmembrane potentials. Optical monitoring systems are well suited to detect such cellular electrical activities in networks of excitable cells and also tissues simultaneously at multiple sites. Here, an exceptionally fast array system (16 x 16 photodiodes, up to 4,000,000 samples per second, 12-bit resolution) for imaging voltage-sensitive dye fluorescence, permitted real time measurements of excitation patterns at a microscopic size scale (256 pixels within an area of 1.8-8 mm2), in rat cardiac muscle in vitro. Results emphasize a recent hypothesis for cardiac impulse conduction, based on cardiac structural complexities, that is contradictory to all continuous cable theory models.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen L. B., Lesher S. Optical monitoring of membrane potential: methods of multisite optical measurement. Soc Gen Physiol Ser. 1986;40:71–99. [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M. Optical measurement of membrane potential. Rev Physiol Biochem Pharmacol. 1978;83:35–88. doi: 10.1007/3-540-08907-1_2. [DOI] [PubMed] [Google Scholar]
- Dillon S., Morad M. A new laser scanning system for measuring action potential propagation in the heart. Science. 1981 Oct 23;214(4519):453–456. doi: 10.1126/science.6974891. [DOI] [PubMed] [Google Scholar]
- Dolber P. C., Spach M. S. Thin collagenous septa in cardiac muscle. Anat Rec. 1987 May;218(1):45–55. doi: 10.1002/ar.1092180109. [DOI] [PubMed] [Google Scholar]
- Fluhler E., Burnham V. G., Loew L. M. Spectra, membrane binding, and potentiometric responses of new charge shift probes. Biochemistry. 1985 Oct 8;24(21):5749–5755. doi: 10.1021/bi00342a010. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Cohen L. B., Lesher S., Boyle M. B. Simultaneous optical monitoring of activity of many neurons in invertebrate ganglia using a 124-element photodiode array. J Neurophysiol. 1981 May;45(5):829–840. doi: 10.1152/jn.1981.45.5.829. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Hildesheim R., Farber I. C., Anglister L. Improved fluorescent probes for the measurement of rapid changes in membrane potential. Biophys J. 1982 Sep;39(3):301–308. doi: 10.1016/S0006-3495(82)84520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Lieke E., Frostig R. D., Gilbert C. D., Wiesel T. N. Functional architecture of cortex revealed by optical imaging of intrinsic signals. 1986 Nov 27-Dec 3Nature. 324(6095):361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Segal M., Kuhnt U., Hildesheim R., Manker A., Anglister L., Freeman J. A. Real-time optical mapping of neuronal activity in vertebrate CNS in vitro and in vivo. Soc Gen Physiol Ser. 1986;40:165–197. [PubMed] [Google Scholar]
- Kauer J. S. Real-time imaging of evoked activity in local circuits of the salamander olfactory bulb. Nature. 1988 Jan 14;331(6152):166–168. doi: 10.1038/331166a0. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Obaid A. L., Salzberg B. M. Optical recording of electrical activity from parallel fibres and other cell types in skate cerebellar slices in vitro. J Physiol. 1987 Dec;393:681–702. doi: 10.1113/jphysiol.1987.sp016848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Dillon S., Weiss J. An acousto-optically steered laser scanning system for measurement of action potential spread in intact heart. Soc Gen Physiol Ser. 1986;40:211–226. [PubMed] [Google Scholar]
- Müller W., Windisch H., Tritthart H. A. Fluorescent styryl dyes applied as fast optical probes of cardiac action potential. Eur Biophys J. 1986;14(2):103–111. doi: 10.1007/BF00263067. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Krauthamer V. Optical measurements of potential changes in axons and processes of neurons of a barnacle ganglion. J Neurosci. 1984 Mar;4(3):659–672. doi: 10.1523/JNEUROSCI.04-03-00659.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama G., Lombardi R., Elson J. Maps of optical action potentials and NADH fluorescence in intact working hearts. Am J Physiol. 1987 Feb;252(2 Pt 2):H384–H394. doi: 10.1152/ajpheart.1987.252.2.H384. [DOI] [PubMed] [Google Scholar]
- Shrager P., Chiu S. Y., Ritchie J. M., Zecevic D., Cohen L. B. Optical recording of action potential propagation in demyelinated frog nerve. Biophys J. 1987 Feb;51(2):351–355. doi: 10.1016/S0006-3495(87)83342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spach M. S., Miller W. T., 3rd, Geselowitz D. B., Barr R. C., Kootsey J. M., Johnson E. A. The discontinuous nature of propagation in normal canine cardiac muscle. Evidence for recurrent discontinuities of intracellular resistance that affect the membrane currents. Circ Res. 1981 Jan;48(1):39–54. doi: 10.1161/01.res.48.1.39. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S. Dye indicators of membrane potential. Annu Rev Biophys Bioeng. 1979;8:47–68. doi: 10.1146/annurev.bb.08.060179.000403. [DOI] [PubMed] [Google Scholar]
- Windisch H., Müller W., Tritthart H. A. Fluorescence monitoring of rapid changes in membrane potential in heart muscle. Biophys J. 1985 Dec;48(6):877–884. doi: 10.1016/S0006-3495(85)83849-2. [DOI] [PMC free article] [PubMed] [Google Scholar]