Abstract
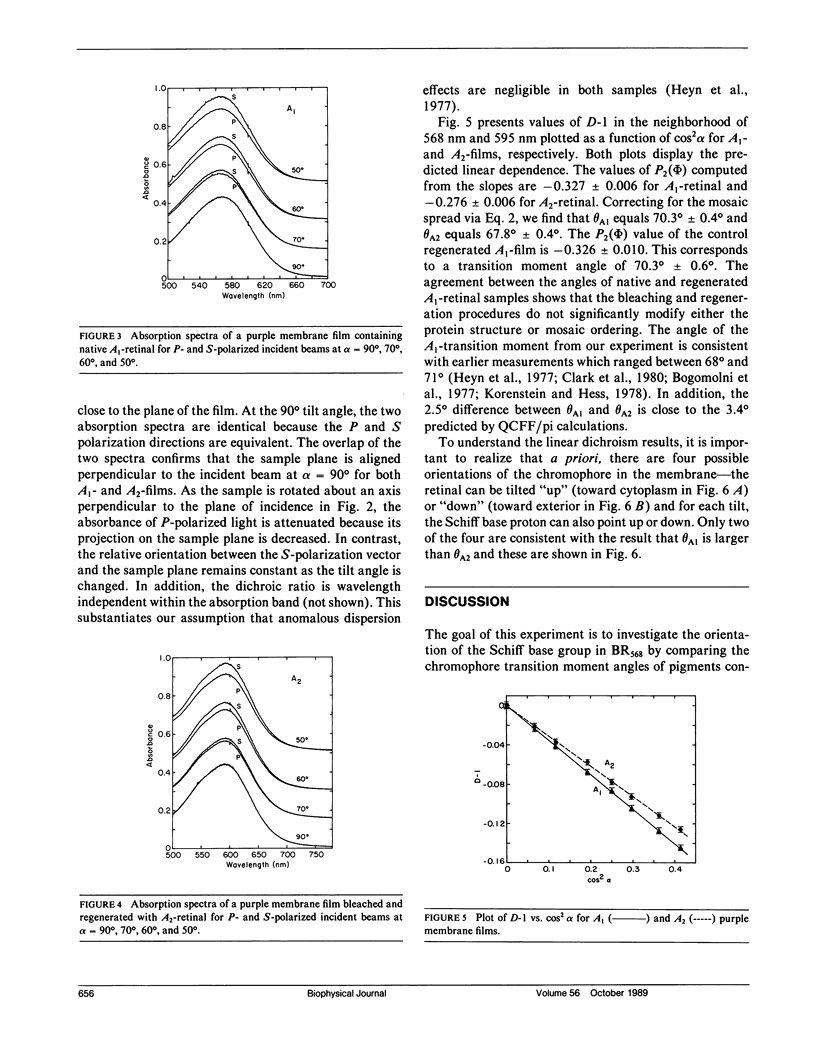
Linear dichroism experiments are performed on light-adapted bacteriorhodopsin (BR568) films containing native retinal (A1) and its 3,4-dehydroretinal (A2) analogue to measure the angle between the chromophore transition dipole moment and the membrane normal. QCFF/pi calculations show that the angle between the transition moment and the long axis of the polyene is changed by 3.4 degrees when the C3-C4 bond is unsaturated. The difference vector between the two transition moments points in the same direction as the Schiff base (N----H) bond for the all-trans BR568 chromophore. Because the plane of the chromophore is perpendicular to the membrane plane, a comparison of the transition moment orientations in the A1- and A2-pigments enables us to determine the orientation of the N----H bond with respect to the absolute chromophore (N----C5 vector) orientation. The angles of the transition moments are 70.3 degrees +/- 0.4 degrees and 67.8 degrees +/- 0.4 degrees for the A1- and A2-pigments, respectively. The fact that the change in the transition moment angle (2.5 degrees) is close to the predicted 3.4 degrees supports the idea that the chromophore plane is nearly perpendicular to the membrane plane. The decreased transition moment angle in the A2-analogue requires that the N----H bond and the N----C5 vector point toward the same membrane surface. Available results indicate that the N----C5 vector points toward the exterior in BR568. With this assignment, we conclude that the N----H bond points toward the exterior surface and its most likely counterion Asp-212. This information makes possible the construction of a computer graphics model for the active site in BR568.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. M., Henderson R., Beckman E., Zemlin F. Images of purple membrane at 2.8 A resolution obtained by cryo-electron microscopy. J Mol Biol. 1988 Aug 5;202(3):585–591. doi: 10.1016/0022-2836(88)90288-4. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Marti T., Stern L. J., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988 Nov 15;27(23):8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Stern L. J., Hackett N. R., Chao B. H., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: I. Tyrosine-185 protonates and deprotonates during the photocycle. Proteins. 1988;3(4):219–229. doi: 10.1002/prot.340030403. [DOI] [PubMed] [Google Scholar]
- Braiman M., Mathies R. Resonance Raman evidence for an all-trans to 13-cis isomerization in the proton-pumping cycle of bacteriorhodopsin. Biochemistry. 1980 Nov 11;19(23):5421–5428. doi: 10.1021/bi00564a042. [DOI] [PubMed] [Google Scholar]
- Clark N. A., Rothschild K. J., Luippold D. A., Simon B. A. Surface-induced lamellar orientation of multilayer membrane arrays. Theoretical analysis and a new method with application to purple membrane fragments. Biophys J. 1980 Jul;31(1):65–96. doi: 10.1016/S0006-3495(80)85041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnest T. N., Roepe P., Braiman M. S., Gillespie J., Rothschild K. J. Orientation of the bacteriorhodopsin chromophore probed by polarized Fourier transform infrared difference spectroscopy. Biochemistry. 1986 Dec 2;25(24):7793–7798. doi: 10.1021/bi00372a002. [DOI] [PubMed] [Google Scholar]
- Ebrey T. G., Becher B., Mao B., Kilbride P., Honig B. Exciton interactions and chromophore orientation in the purple membrane. J Mol Biol. 1977 May 25;112(3):377–397. doi: 10.1016/s0022-2836(77)80188-5. [DOI] [PubMed] [Google Scholar]
- Engelhard M., Gerwert K., Hess B., Kreutz W., Siebert F. Light-driven protonation changes of internal aspartic acids of bacteriorhodopsin: an investigation by static and time-resolved infrared difference spectroscopy using [4-13C]aspartic acid labeled purple membrane. Biochemistry. 1985 Jan 15;24(2):400–407. doi: 10.1021/bi00323a024. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Fodor S. P., Ames J. B., Gebhard R., van den Berg E. M., Stoeckenius W., Lugtenburg J., Mathies R. A. Chromophore structure in bacteriorhodopsin's N intermediate: implications for the proton-pumping mechanism. Biochemistry. 1988 Sep 6;27(18):7097–7101. doi: 10.1021/bi00418a064. [DOI] [PubMed] [Google Scholar]
- Harbison G. S., Herzfeld J., Griffin R. G. Solid-state nitrogen-15 nuclear magnetic resonance study of the Schiff base in bacteriorhodopsin. Biochemistry. 1983 Jan 4;22(1):1–4. doi: 10.1021/bi00270a600. [DOI] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Courtin J. M., Lugtenburg J., Herzfeld J., Mathies R. A., Griffin R. G. Solid-state 13C NMR detection of a perturbed 6-s-trans chromophore in bacteriorhodopsin. Biochemistry. 1985 Nov 19;24(24):6955–6962. doi: 10.1021/bi00345a031. [DOI] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Winkel C., Lugtenburg J., Herzfeld J., Mathies R., Griffin R. G. Dark-adapted bacteriorhodopsin contains 13-cis, 15-syn and all-trans, 15-anti retinal Schiff bases. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1706–1709. doi: 10.1073/pnas.81.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. B., Stroud R. M. Projected structure of purple membrane determined to 3.7 A resolution by low temperature electron microscopy. J Mol Biol. 1981 Sep 25;151(3):491–517. doi: 10.1016/0022-2836(81)90007-3. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Cherry R. J., Müller U. Transient and linear dichroism studies on bacteriorhodopsin: determination of the orientation of the 568 nm all-trans retinal chromophore. J Mol Biol. 1977 Dec 15;117(3):607–620. doi: 10.1016/0022-2836(77)90060-2. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Westerhausen J., Wallat I., Seiff F. High-sensitivity neutron diffraction of membranes: Location of the Schiff base end of the chromophore of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2146–2150. doi: 10.1073/pnas.85.7.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz M., Drachev L. A., Mogi T., Otto H., Kaulen A. D., Heyn M. P., Skulachev V. P., Khorana H. G. Replacement of aspartic acid-96 by asparagine in bacteriorhodopsin slows both the decay of the M intermediate and the associated proton movement. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2167–2171. doi: 10.1073/pnas.86.7.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. Y., Lewis A. Determination of the absolute orientation of the retinylidene chromophore in purple membrane by a second-harmonic interference technique. Biophys J. 1989 May;55(5):835–842. doi: 10.1016/S0006-3495(89)82883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. S., Radhakrishnan R., Bayley H., Khorana H. G. Orientation of retinal in bacteriorhodopsin as studied by cross-linking using a photosensitive analog of retinal. J Biol Chem. 1982 Nov 25;257(22):13616–13623. [PubMed] [Google Scholar]
- Korenstein R., Hess B. Immobilization of bacteriorhodopsin and orientation of its transition moment in purple membrane. FEBS Lett. 1978 May 1;89(1):15–20. doi: 10.1016/0014-5793(78)80512-2. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Bogomolni R. A., Stoeckenius W. Photoconversion from the light-adapted to the dark-adapted state of bacteriorhodopsin. Biophys J. 1985 Aug;48(2):201–208. doi: 10.1016/S0006-3495(85)83773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. L., Ormos P., Eisenstein L., Govindjee R., Konno K., Nakanishi K. Deprotonation of tyrosines in bacteriorhodopsin as studied by Fourier transform infrared spectroscopy with deuterium and nitrate labeling. Biochemistry. 1987 Dec 15;26(25):8327–8331. doi: 10.1021/bi00399a045. [DOI] [PubMed] [Google Scholar]
- Marcus M. A., Lewis A., Crespi H. Physiological and structural investigations of bacteriorhodopsin analogs. Biochem Biophys Res Commun. 1977 Sep 23;78(2):669–675. doi: 10.1016/0006-291x(77)90231-5. [DOI] [PubMed] [Google Scholar]
- Mathies R. A., Brito Cruz C. H., Pollard W. T., Shank C. V. Direct observation of the femtosecond excited-state cis-trans isomerization in bacteriorhodopsin. Science. 1988 May 6;240(4853):777–779. doi: 10.1126/science.3363359. [DOI] [PubMed] [Google Scholar]
- Mogi T., Stern L. J., Hackett N. R., Khorana H. G. Bacteriorhodopsin mutants containing single tyrosine to phenylalanine substitutions are all active in proton translocation. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5595–5599. doi: 10.1073/pnas.84.16.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi T., Stern L. J., Marti T., Chao B. H., Khorana H. G. Aspartic acid substitutions affect proton translocation by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4148–4152. doi: 10.1073/pnas.85.12.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otomo J., Tomioka A., Kinosita K., Miyata H., Takenaka Y., Kouyama T., Ikegami A. Chromophore of Bacteriorhodopsin is Closer to the Cytoplasmic Surface of Purple Membrane: Fluorescence Energy Transfer on Oriented Membrane Sheets. Biophys J. 1988 Jul;54(1):57–64. doi: 10.1016/s0006-3495(88)82930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepe P., Ahl P. L., Das Gupta S. K., Herzfeld J., Rothschild K. J. Tyrosine and carboxyl protonation changes in the bacteriorhodopsin photocycle. 1. M412 and L550 intermediates. Biochemistry. 1987 Oct 20;26(21):6696–6707. doi: 10.1021/bi00395a020. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Clark N. A. Polarized infrared spectroscopy of oriented purple membrane. Biophys J. 1979 Mar;25(3):473–487. doi: 10.1016/S0006-3495(79)85317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Roepe P., Lugtenburg J., Pardoen J. A. Fourier transform infrared evidence for Schiff base alteration in the first step of the bacteriorhodopsin photocycle. Biochemistry. 1984 Dec 4;23(25):6103–6109. doi: 10.1021/bi00320a031. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Myers A. B., Pardoen J. A., Winkel C., Mulder P. P., Lugtenburg J., Mathies R. Determination of retinal Schiff base configuration in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2055–2059. doi: 10.1073/pnas.81.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., McCain D. A., Nakanishi K., Okabe M., Shimizu N., Rodman H., Honig B., Bogomolni R. A. Chromophore/protein interaction in bacterial sensory rhodopsin and bacteriorhodopsin. Biophys J. 1986 Feb;49(2):479–483. doi: 10.1016/S0006-3495(86)83657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Tokunaga F., Ebrey T. The blue membrane: the 3-dehydroretinal-based artificial pigment of the purple membrane. Biochemistry. 1978 May 16;17(10):1915–1922. doi: 10.1021/bi00603a018. [DOI] [PubMed] [Google Scholar]
- Tokunaga F., Govindjee R., Ebrey T. G. Synthetic pigment analogues of the purple membrane protein. Biophys J. 1977 Aug;19(2):191–198. doi: 10.1016/S0006-3495(77)85580-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A., Karplus M. Calculation of pi-pi excited state conformations and vibronic structure of retinal and related molecules. J Am Chem Soc. 1974 Sep 4;96(18):5677–5689. doi: 10.1021/ja00825a001. [DOI] [PubMed] [Google Scholar]


