Abstract
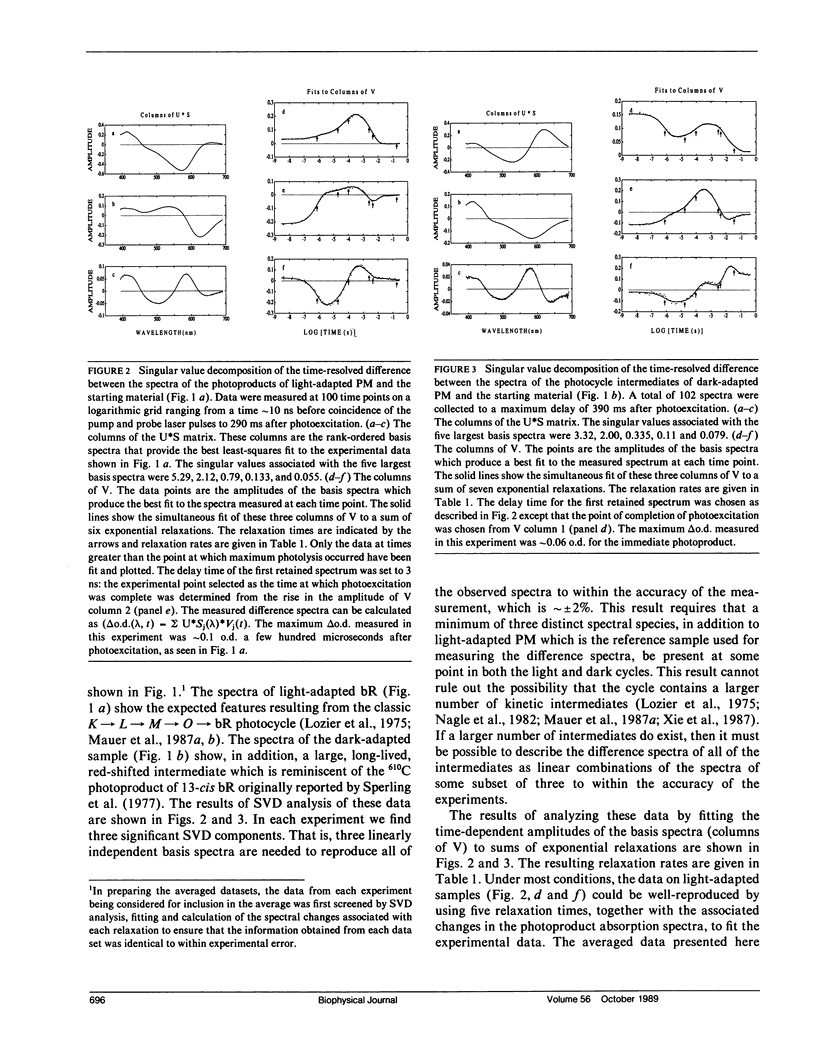
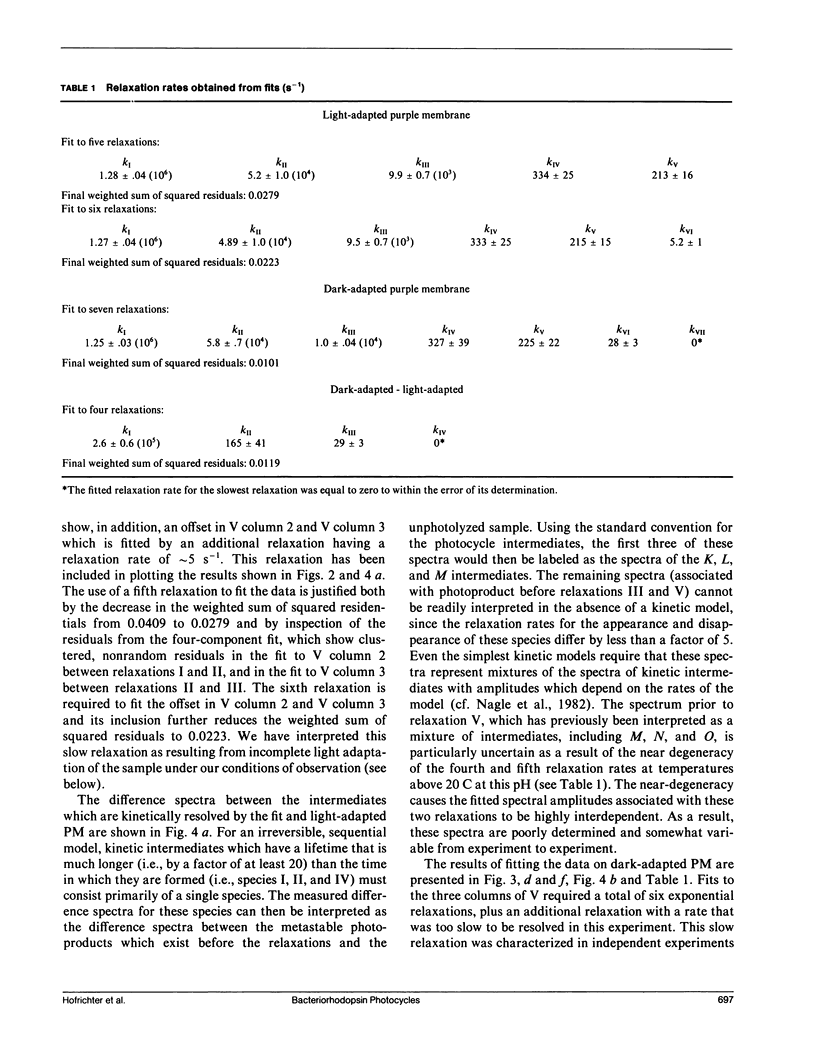
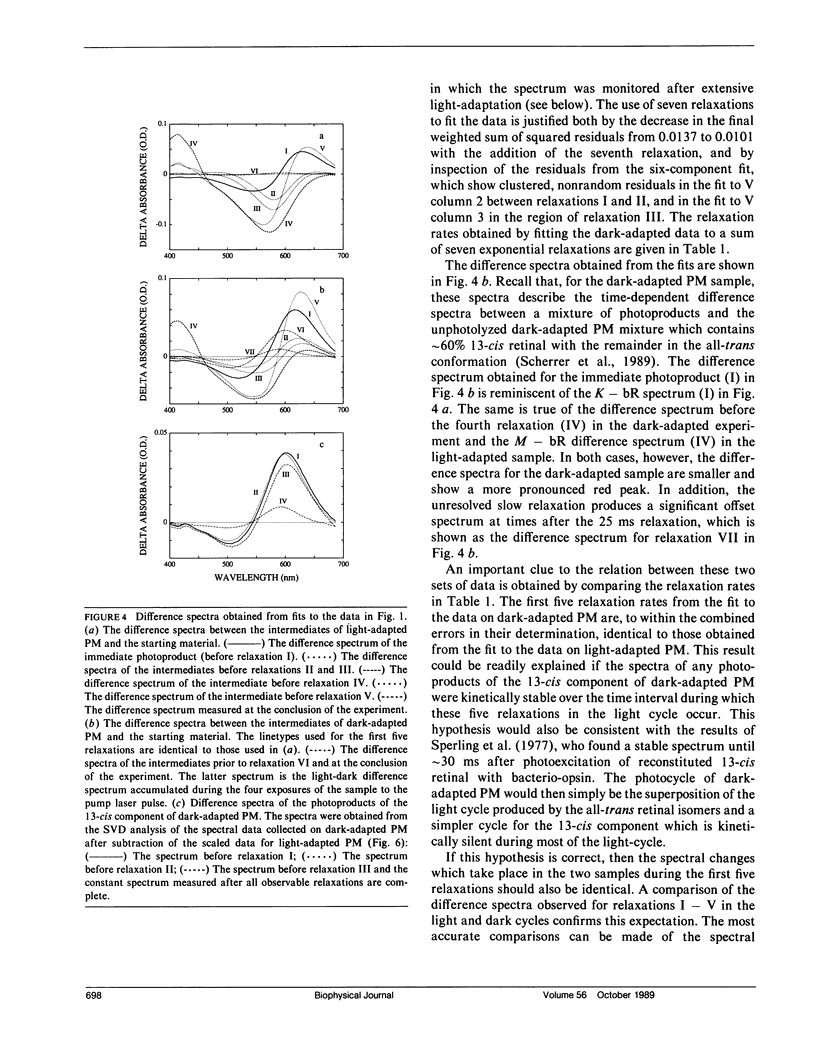
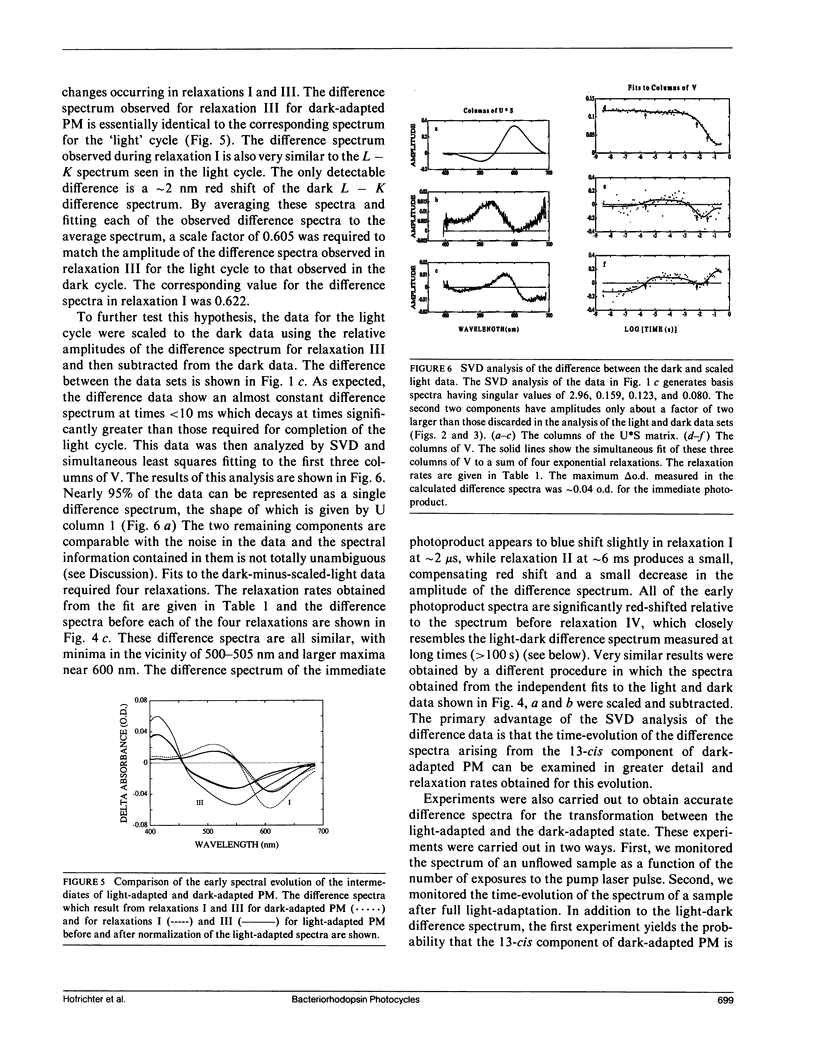
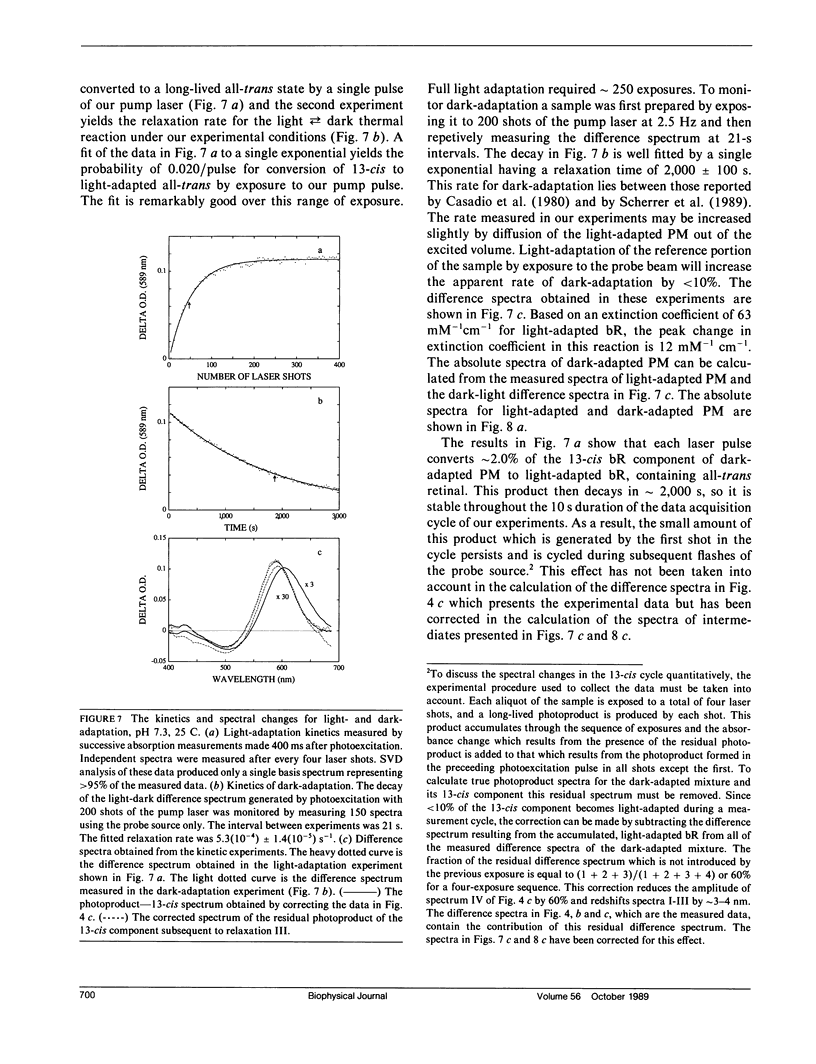
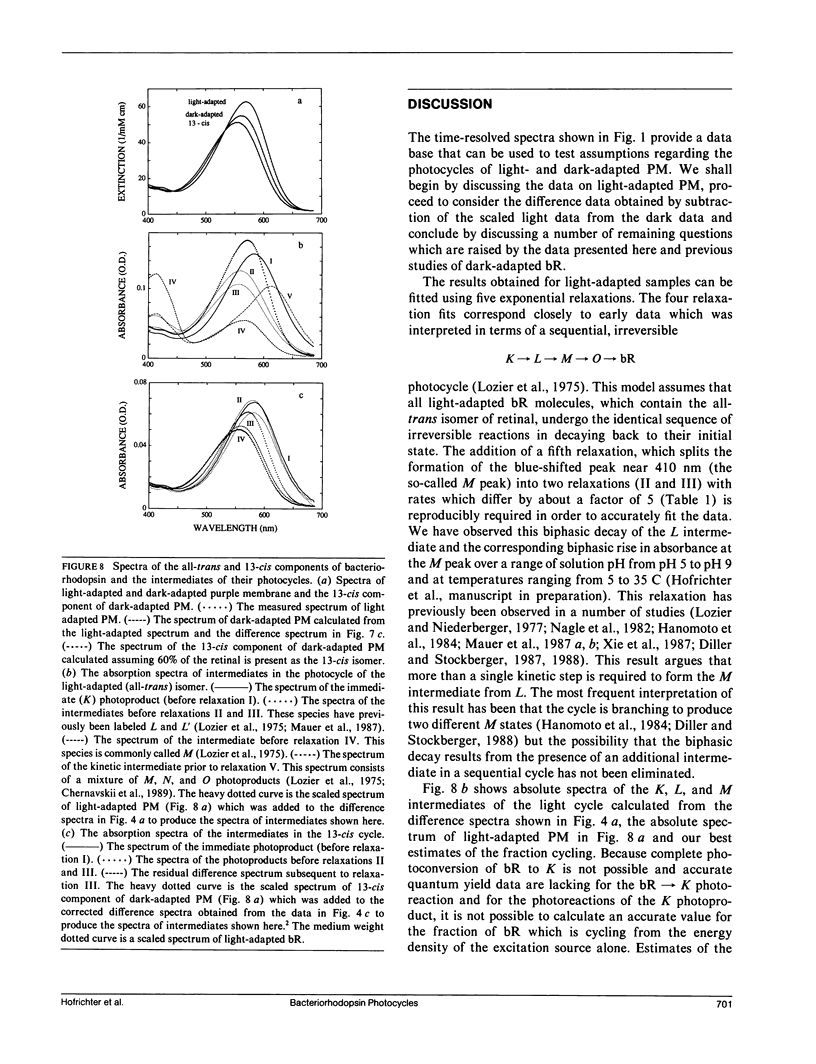
Nanosecond time-resolved absorption spectra have been measured throughout the photocycle of bacteriorhodopsin in both light-adapted and dark-adapted purple membrane (PM). The data from dark-adapted samples are interpretable as the superposition of two photocycles arising independently from the all-trans and 13-cis retinal isomers that coexist in the dark-adapted state. The presence of a photocycle in dark-adapted PM which is indistinguishable from that observed for light-adapted PM under the same experimental conditions is demonstrated by the observation of the same five relaxation rates associated with essentially identical changes in the photoproduct spectra. This cycle is attributed to the all-trans component. The cycle of the 13-cis component is revealed by scaling the data measured for the light-adapted sample and subtracting it from the data on the dark-adapted mixture. At times less than 1 ms, the resulting difference spectra are nearly time-independent. The peak of the difference spectrum is near 600 nm, although there appears to be a slight (approximately 2 nm) blue-shift in the first few microseconds. Subsequently the amplitude of this spectrum decays and the peak of the difference spectrum shifts in two relaxations. Most of the amplitude of the photoproduct difference spectrum (approximately 80%) decays in a single relaxation having a time constant of approximately 35 ms. The difference spectrum remaining after this relaxation peaks at approximately 590 nm and is indistinguishable from the classical light-dark difference spectrum, which we find, in experiments performed on a much longer time scale, to peak at 588 nm. The decay of this remaining photo-product is not resolvable in the nanosecond kinetic experiments, but dark adaptation of a completely light-adapted sample is found to occur exponentially with a relaxation time of approximately 2,000 s under the conditions of our experiments.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birge R. R. Photophysics of light transduction in rhodopsin and bacteriorhodopsin. Annu Rev Biophys Bioeng. 1981;10:315–354. doi: 10.1146/annurev.bb.10.060181.001531. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A., Baker R. A., Lozier R. H., Stoeckenius W. Action spectrum and quantum efficiency for proton pumping in Halobacterium halobium. Biochemistry. 1980 May 13;19(10):2152–2159. doi: 10.1021/bi00551a024. [DOI] [PubMed] [Google Scholar]
- Braiman M., Mathies R. Resonance Raman spectra of bacteriorhodopsin's primary photoproduct: evidence for a distorted 13-cis retinal chromophore. Proc Natl Acad Sci U S A. 1982 Jan;79(2):403–407. doi: 10.1073/pnas.79.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio R., Gutowitz H., Mowery P., Taylor M., Stoeckenius W. Light-dark adaptation of bacteriorhodopsin in triton-treated purple membrane. Biochim Biophys Acta. 1980 Mar 7;590(1):13–23. doi: 10.1016/0005-2728(80)90142-5. [DOI] [PubMed] [Google Scholar]
- Fodor S. P., Ames J. B., Gebhard R., van den Berg E. M., Stoeckenius W., Lugtenburg J., Mathies R. A. Chromophore structure in bacteriorhodopsin's N intermediate: implications for the proton-pumping mechanism. Biochemistry. 1988 Sep 6;27(18):7097–7101. doi: 10.1021/bi00418a064. [DOI] [PubMed] [Google Scholar]
- Hanamoto J. H., Dupuis P., El-Sayed M. A. On the protein (tyrosine)-chromophore (protonated Schiff base) coupling in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7083–7087. doi: 10.1073/pnas.81.22.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Winkel C., Lugtenburg J., Herzfeld J., Mathies R., Griffin R. G. Dark-adapted bacteriorhodopsin contains 13-cis, 15-syn and all-trans, 15-anti retinal Schiff bases. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1706–1709. doi: 10.1073/pnas.81.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Henry E. R., Sommer J. H., Deutsch R., Ikeda-Saito M., Yonetani T., Eaton W. A. Nanosecond optical spectra of iron-cobalt hybrid hemoglobins: geminate recombination, conformational changes, and intersubunit communication. Biochemistry. 1985 May 21;24(11):2667–2679. doi: 10.1021/bi00332a012. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Sommer J. H., Henry E. R., Eaton W. A. Nanosecond absorption spectroscopy of hemoglobin: elementary processes in kinetic cooperativity. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2235–2239. doi: 10.1073/pnas.80.8.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisky O., Goldschmidt C. R., Ottolenghi M. On the photocycle and light adaptation of dark-adapted bacteriorhodopsin. Biophys J. 1977 Aug;19(2):185–189. doi: 10.1016/S0006-3495(77)85579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A., Iwasa T., Yoshizawa T. Isomeric composition of retinal chromophore in dark-adapted bacteriorhodopsin. J Biochem. 1977 Dec;82(6):1599–1604. doi: 10.1093/oxfordjournals.jbchem.a131855. [DOI] [PubMed] [Google Scholar]
- Murray L. P., Hofrichter J., Henry E. R., Ikeda-Saito M., Kitagishi K., Yonetani T., Eaton W. A. The effect of quaternary structure on the kinetics of conformational changes and nanosecond geminate rebinding of carbon monoxide to hemoglobin. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2151–2155. doi: 10.1073/pnas.85.7.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Parodi L. A., Lozier R. H. Procedure for testing kinetic models of the photocycle of bacteriorhodopsin. Biophys J. 1982 May;38(2):161–174. doi: 10.1016/S0006-3495(82)84543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Meentzen M., Schuhmann L. Reversible dissociation of the purple complex in bacteriorhodopsin and identification of 13-cis and all-trans-retinal as its chromophores. Eur J Biochem. 1973 Dec 17;40(2):453–463. doi: 10.1111/j.1432-1033.1973.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Roepe P. D., Ahl P. L., Herzfeld J., Lugtenburg J., Rothschild K. J. Tyrosine protonation changes in bacteriorhodopsin. A Fourier transform infrared study of BR548 and its primary photoproduct. J Biol Chem. 1988 Apr 15;263(11):5110–5117. [PubMed] [Google Scholar]
- Smith S. O., Hornung I., van der Steen R., Pardoen J. A., Braiman M. S., Lugtenburg J., Mathies R. A. Are C14-C15 single bond isomerizations of the retinal chromophore involved in the proton-pumping mechanism of bacteriorhodopsin? Proc Natl Acad Sci U S A. 1986 Feb;83(4):967–971. doi: 10.1073/pnas.83.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. O., Myers A. B., Pardoen J. A., Winkel C., Mulder P. P., Lugtenburg J., Mathies R. Determination of retinal Schiff base configuration in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2055–2059. doi: 10.1073/pnas.81.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling W., Carl P., Rafferty Ch, Dencher N. A. Photochemistry and dark equilibrium of retinal isomers and bacteriorhodopsin isomers. Biophys Struct Mech. 1977 Jun 29;3(2):79–94. doi: 10.1007/BF00535798. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Xie A. H., Nagle J. F., Lozier R. H. Flash spectroscopy of purple membrane. Biophys J. 1987 Apr;51(4):627–635. doi: 10.1016/S0006-3495(87)83387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


