Abstract
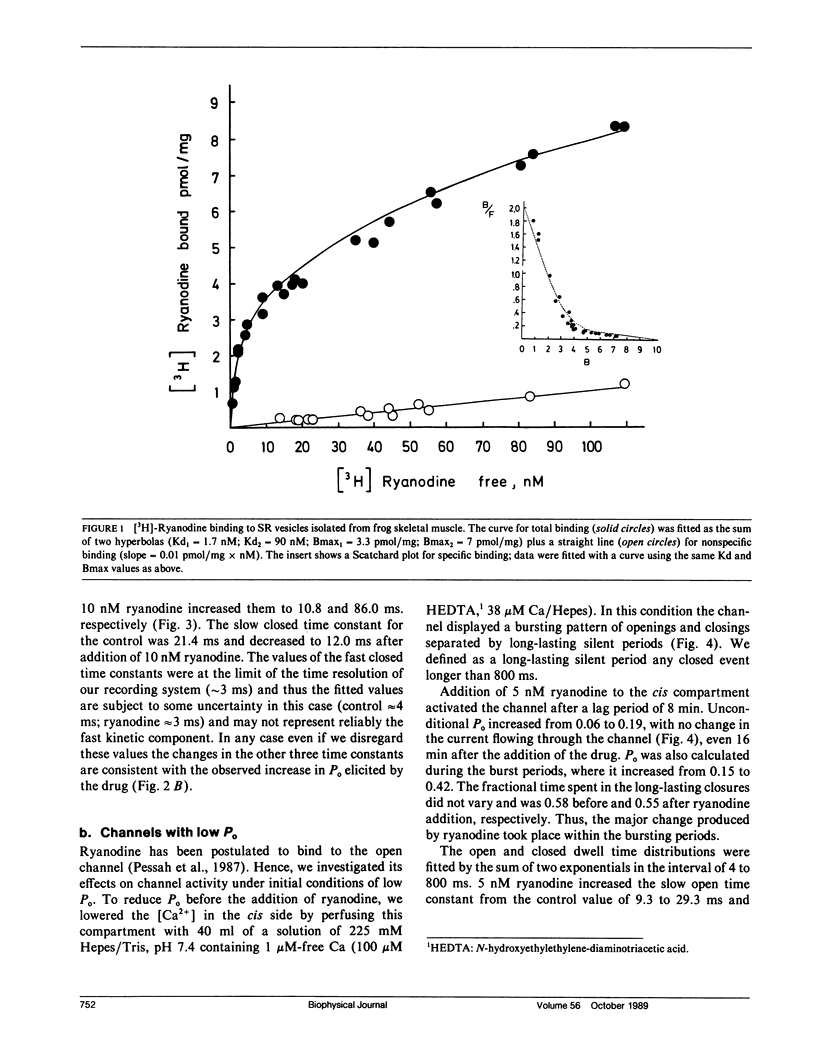
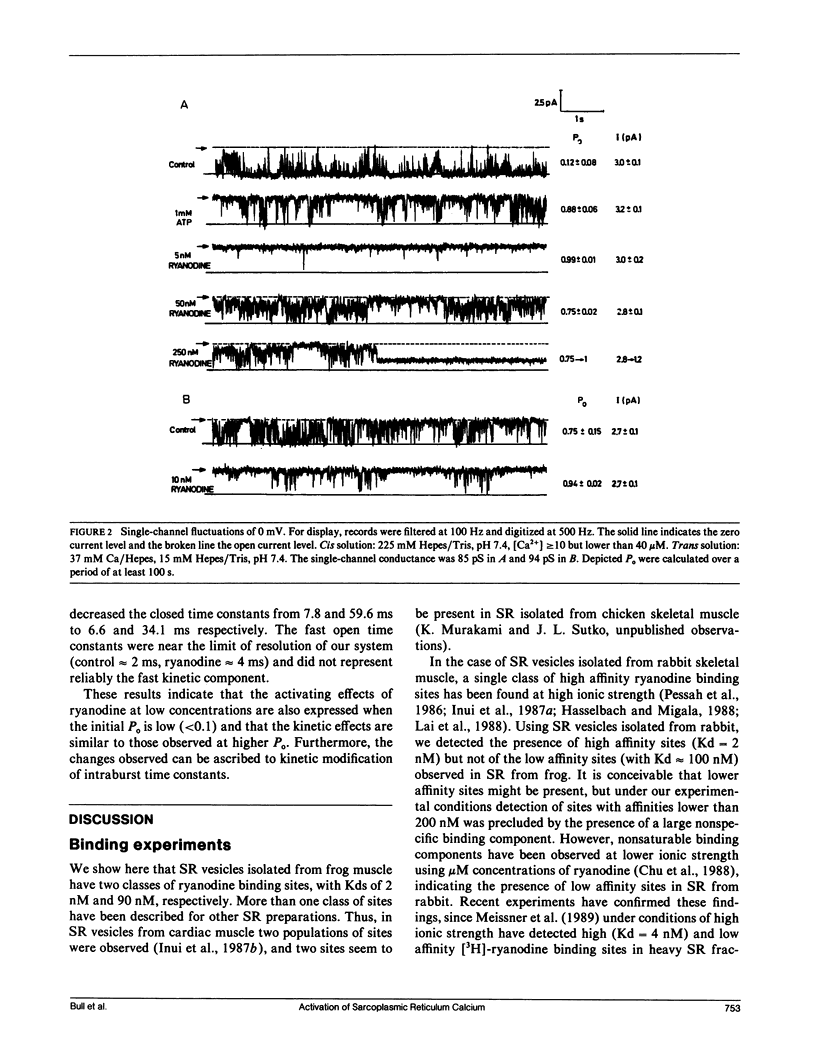
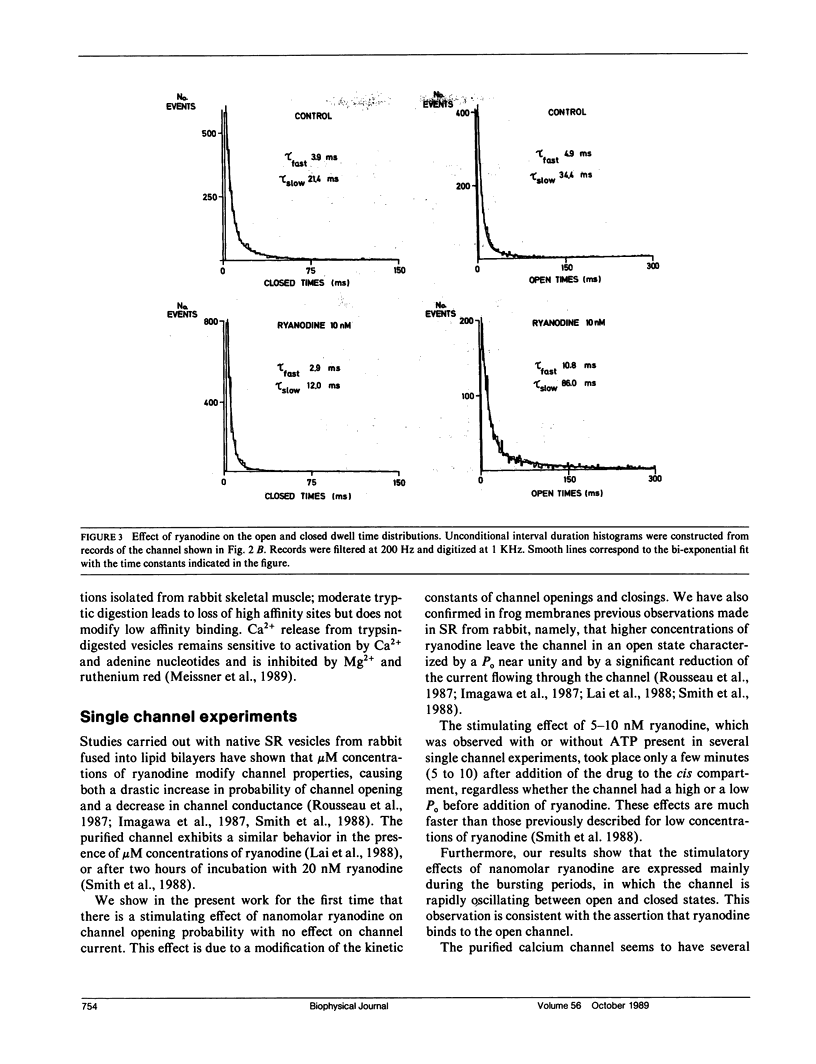
Sarcoplasmic reticulum vesicles isolated from fast-twitch frog skeletal muscle presented two classes of binding sites for ryanodine, one of high affinity (Kd1 = 1.7 nM, Bmax1 = 3.3 pmol per mg) and a second class with lower affinity (Kd2 = 90 nM, Bmax2 = 7.0 pmol per milligram). The calcium channels present in the sarcoplasmic reticulum membranes were studied in vesicles fused into lipid bilayers. Low concentrations of ryanodine (5 to 10 nM) activated a large conductance calcium channel after a short delay (5 to 10 min). The activation, which could be elicited from conditions of high or low fractional open time, was characterized by an increase in channel fractional open time without a change in conductance. The open and closed dwell time distributions were fitted with the sum of two exponentials in the range of 4 to 800 ms. The activating effect of ryanodine was due to an increase of both open time constants and a concomitant decrease in the closed time constants. Under conditions of low fractional open time (less than 0.1), the time spent in long closed periods (greater than 800 ms) between bursts was not affected by ryanodine. Higher concentrations of ryanodine (250 nM) locked the channel in a lower conductance level (approximately 40%) with a fractional open time near unity. These results suggest that the activating effects of nanomolar concentrations of ryanodine may arise from drug binding to high affinity sites. The expression of the lower conductance state obtained with higher concentrations of ryanodine may be associated with the low affinity binding sites observed in frog sarcoplasmic reticulum.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell K. P., Knudson C. M., Imagawa T., Leung A. T., Sutko J. L., Kahl S. D., Raab C. R., Madson L. Identification and characterization of the high affinity [3H]ryanodine receptor of the junctional sarcoplasmic reticulum Ca2+ release channel. J Biol Chem. 1987 May 15;262(14):6460–6463. [PubMed] [Google Scholar]
- Chu A., Sumbilla C., Scales D., Piazza A., Inesi G. Trypsin digestion of junctional sarcoplasmic reticulum vesicles. Biochemistry. 1988 Apr 19;27(8):2827–2833. doi: 10.1021/bi00408a025. [DOI] [PubMed] [Google Scholar]
- Fairhurst A. S., Hasselbach W. Calcium efflux from a heavy sarcotubular fraction. Effects of ryanodine, caffeine and magnesium. Eur J Biochem. 1970 Apr;13(3):504–509. doi: 10.1111/j.1432-1033.1970.tb00953.x. [DOI] [PubMed] [Google Scholar]
- Fernandez J. L., Rosemblatt M., Hidalgo C. Highly purified sarcoplasmic reticulum vesicles are devoid of Ca2+-independent ('basal') ATPase activity. Biochim Biophys Acta. 1980 Jul;599(2):552–568. doi: 10.1016/0005-2736(80)90199-6. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Ogunbunmi E. M., Dixon M. C., Fleer E. A. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C. STUDIES OF THE TRIAD : I. Structure of the Junction in Frog Twitch Fibers. J Cell Biol. 1970 Nov 1;47(2):488–499. doi: 10.1083/jcb.47.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselbach W., Migala A. Interaction of ryanodine with the calcium releasing system of sarcoplasmic reticulum vesicles. Z Naturforsch C. 1988 Jan-Feb;43(1-2):140–148. doi: 10.1515/znc-1988-1-225. [DOI] [PubMed] [Google Scholar]
- Hymel L., Inui M., Fleischer S., Schindler H. Purified ryanodine receptor of skeletal muscle sarcoplasmic reticulum forms Ca2+-activated oligomeric Ca2+ channels in planar bilayers. Proc Natl Acad Sci U S A. 1988 Jan;85(2):441–445. doi: 10.1073/pnas.85.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa T., Smith J. S., Coronado R., Campbell K. P. Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel. J Biol Chem. 1987 Dec 5;262(34):16636–16643. [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J Biol Chem. 1987 Nov 15;262(32):15637–15642. [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987 Feb 5;262(4):1740–1747. [PubMed] [Google Scholar]
- Jenden D. J., Fairhurst A. S. The pharmacology of ryanodine. Pharmacol Rev. 1969 Mar;21(1):1–25. [PubMed] [Google Scholar]
- Katz N. L., Ingenito A., Procita L. Ryanodine-induced contractile failure of skeletal muscle. J Pharmacol Exp Ther. 1970 Feb;171(2):242–248. [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr, Schlatterer R. G., Nicar M., Campbell K. P., Sutko J. L. The effects of ryanodine on passive calcium fluxes across sarcoplasmic reticulum membranes. J Biol Chem. 1987 Feb 25;262(6):2711–2718. [PubMed] [Google Scholar]
- Ma J., Fill M., Knudson C. M., Campbell K. P., Coronado R. Ryanodine receptor of skeletal muscle is a gap junction-type channel. Science. 1988 Oct 7;242(4875):99–102. doi: 10.1126/science.2459777. [DOI] [PubMed] [Google Scholar]
- Meissner G., Rousseau E., Lai F. A. Structural and functional correlation of the trypsin-digested Ca2+ release channel of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1989 Jan 25;264(3):1715–1722. [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986 May 15;261(14):6300–6306. [PubMed] [Google Scholar]
- Pessah I. N., Francini A. O., Scales D. J., Waterhouse A. L., Casida J. E. Calcium-ryanodine receptor complex. Solubilization and partial characterization from skeletal muscle junctional sarcoplasmic reticulum vesicles. J Biol Chem. 1986 Jul 5;261(19):8643–8648. [PubMed] [Google Scholar]
- Pessah I. N., Stambuk R. A., Casida J. E. Ca2+-activated ryanodine binding: mechanisms of sensitivity and intensity modulation by Mg2+, caffeine, and adenine nucleotides. Mol Pharmacol. 1987 Mar;31(3):232–238. [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987 Sep;253(3 Pt 1):C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature. 1985 Aug 1;316(6027):446–449. doi: 10.1038/316446a0. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum. Activation by Ca2+ and ATP and modulation by Mg2+. J Gen Physiol. 1986 Nov;88(5):573–588. doi: 10.1085/jgp.88.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Imagawa T., Ma J., Fill M., Campbell K. P., Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988 Jul;92(1):1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko J. L., Ito K., Kenyon J. L. Ryanodine: a modifier of sarcoplasmic reticulum calcium release in striated muscle. Fed Proc. 1985 Dec;44(15):2984–2988. [PubMed] [Google Scholar]
- Suárez-Isla B. A., Irribarra V., Oberhauser A., Larralde L., Bull R., Hidalgo C., Jaimovich E. Inositol (1,4,5)-trisphosphate activates a calcium channel in isolated sarcoplasmic reticulum membranes. Biophys J. 1988 Oct;54(4):737–741. doi: 10.1016/S0006-3495(88)83009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


