Abstract
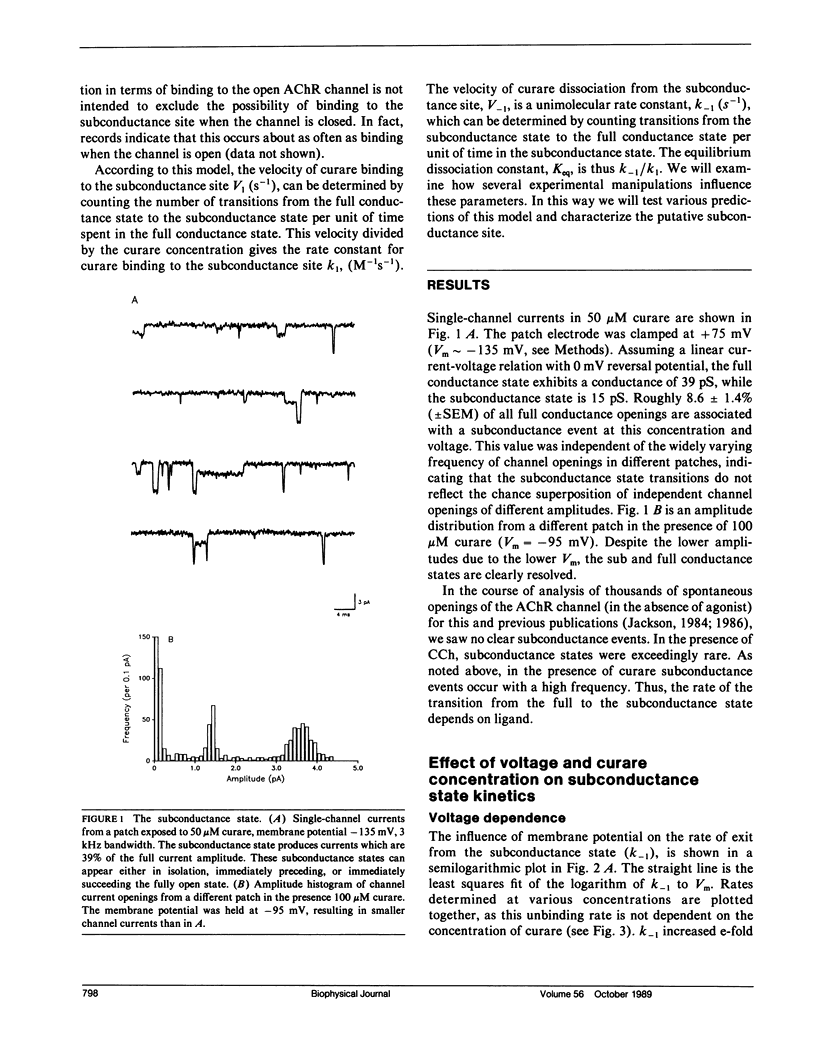
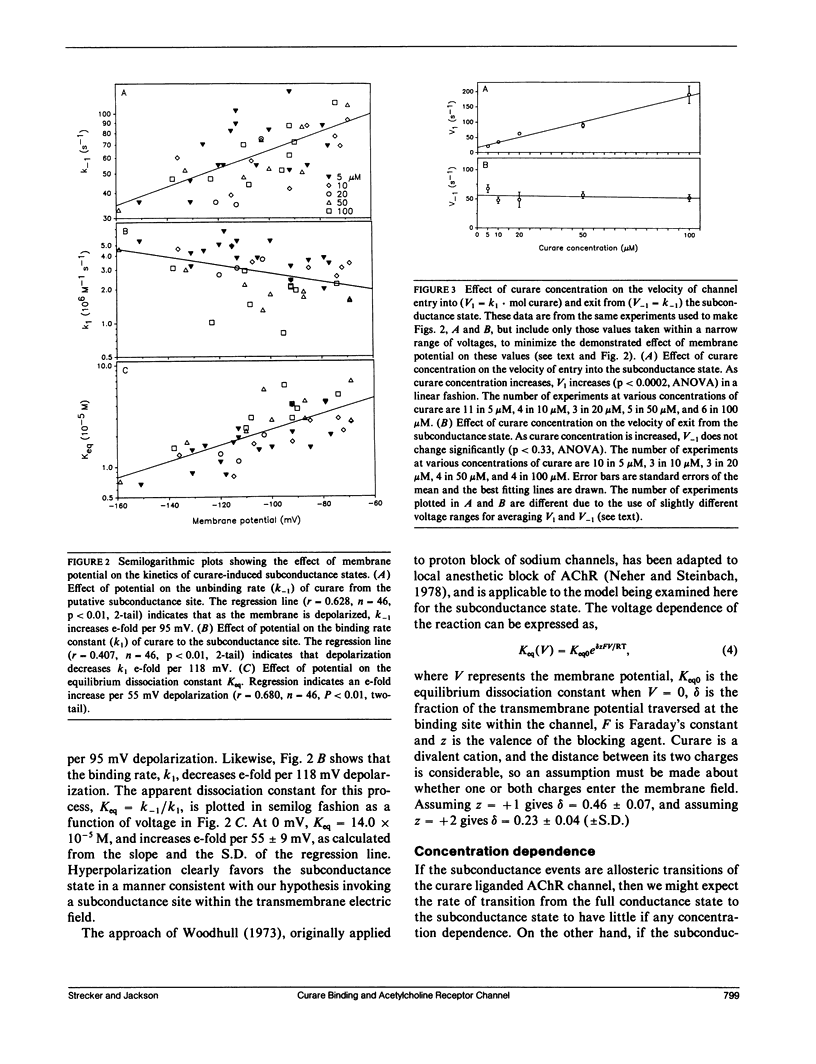
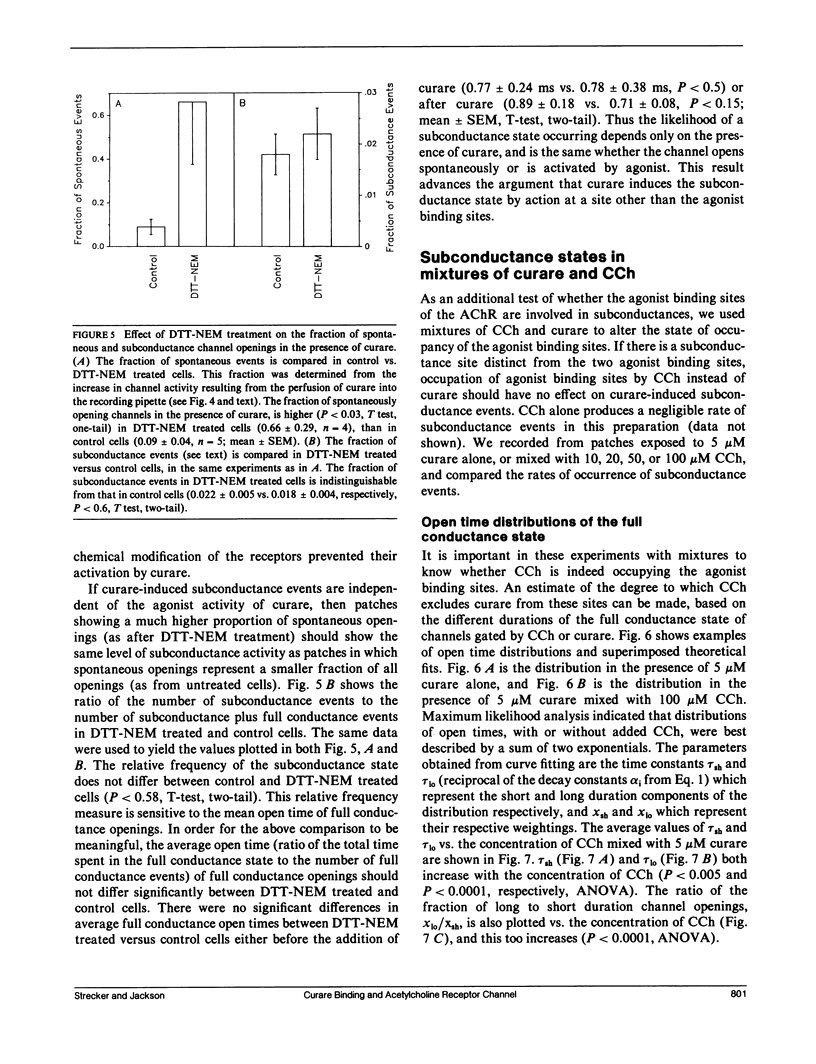
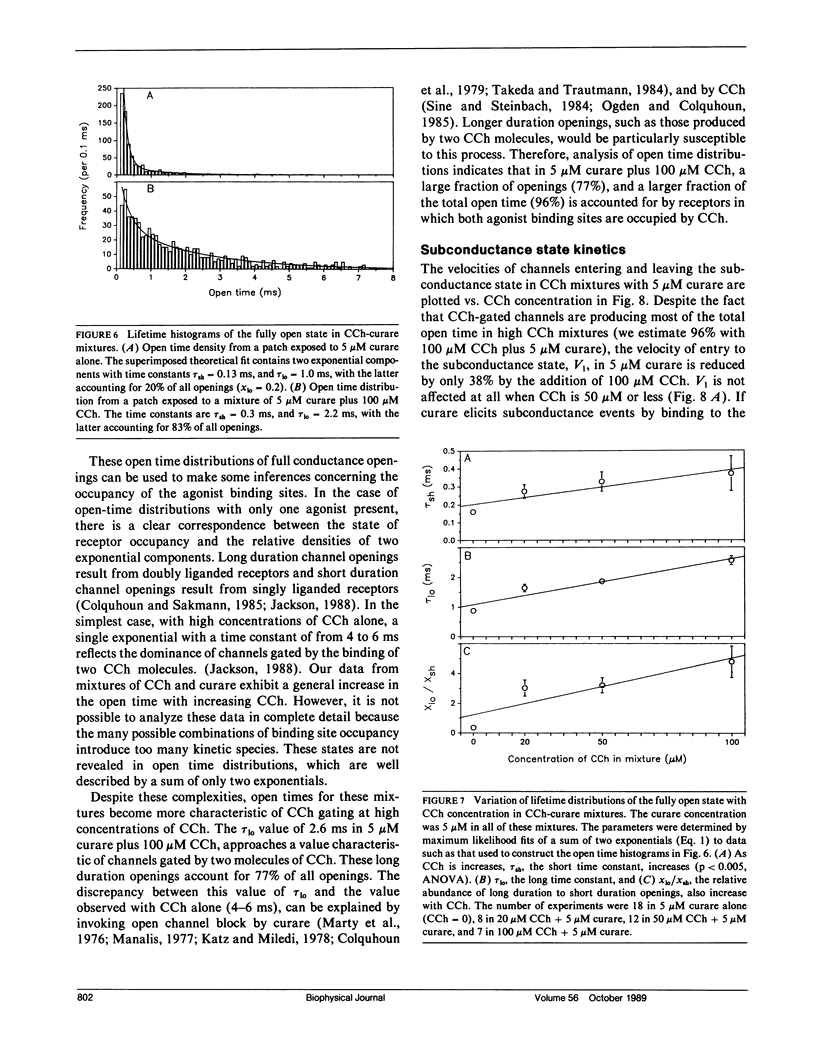
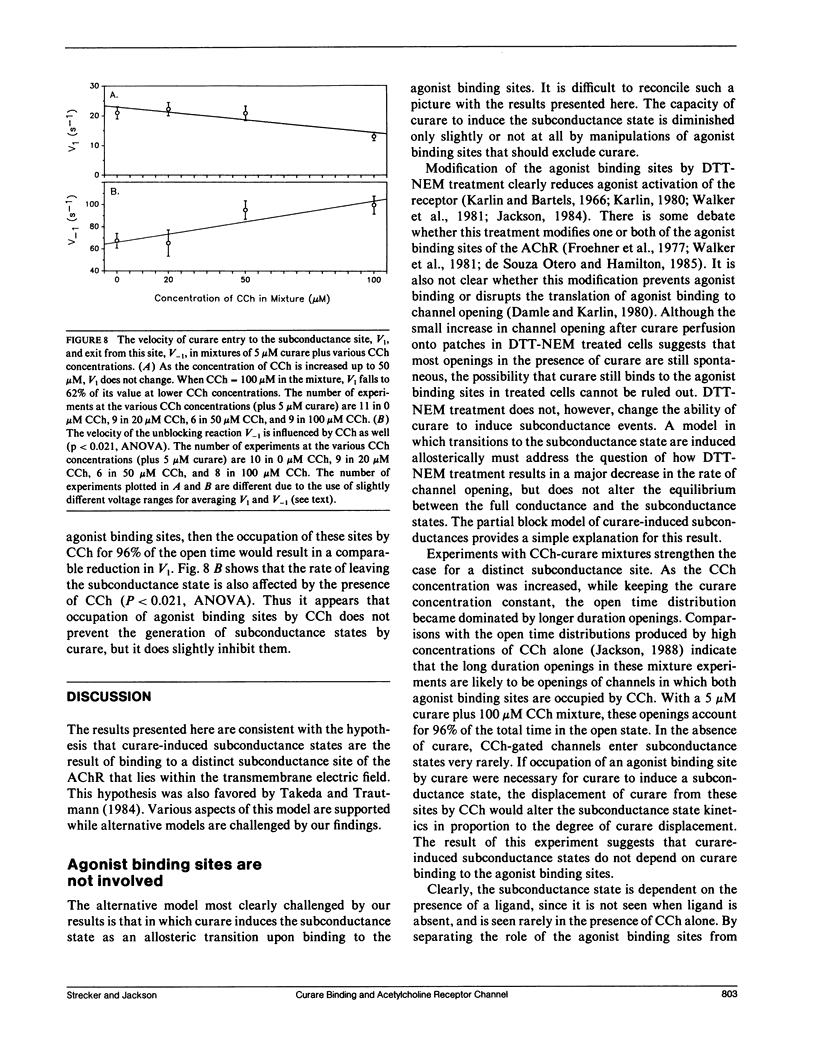
The curare-induced subconductance state of the nicotinic acetylcholine receptor (AChR) of mouse skeletal muscle was examined using the patch-clamp technique. Two mechanisms for the generation of subconductance states were considered. One of these mechanisms entails allosteric induction of a distinct channel conformation through the binding of curare to the agonist binding site. The other mechanism entails the binding of curare to a different site on the protein. Occupation of this site would then limit the flow of ions through the channel. The voltage dependence and concentration dependence of subconductance state kinetics are consistent with curare binding to a site within the channel. The first order rate constant for binding is 1.2 X 10(6) M-1s-1 at 0 mV, and increases e-fold per 118 mV of membrane hyperpolarization. The rate of curare dissociation from this site is 1.9 X 10(2)s-1 at 0 mV, and decreases e-fold per 95 mV hyperpolarization. The equilibrium constant is 1.4 X 10(-4) M at 0 mV, and decreases e-fold per 55 mV hyperpolarization. This voltage dependence suggests that the fraction of the transmembrane potential traversed by curare in binding to this site is 0.46 or 0.23, depending on whether one assumes that one or both charges of curare sense the electric field. Successive reduction and alkylation of the AChR agonist binding sites with dithiothreitol (DTT) and N-ethyl maleimide (NEM), a treatment which results in the loss of responsiveness of the AChR to agonists, produced no change in curare-induced subconductance events, despite the fact that after this treatment most of the channel openings occurred spontaneously. Mixtures of high concentrations of carbamylcholine (CCh) with a low concentration of curare, which produce channel openings gated predominantly by CCH, resulted in subconductance state kinetics similar to those seen in curare alone at the same concentration. Thus displacement by CCh of curare from the agonist binding sites does not prevent curare from inducing subconductances. The results presented here support the hypothesis that curare induces subconductance states by binding to a site on the receptor other than the agonist binding sites, possibly within the channel pore. It is the occupation of this site by curare that limits the flow of ions through an otherwise fully opened channel.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Sakmann B. Decamethonium both opens and blocks endplate channels. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2994–2998. doi: 10.1073/pnas.75.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A., Sachs F. Flickering of a nicotinic ion channel to a subconductance state. Biophys J. 1983 Apr;42(1):1–10. doi: 10.1016/S0006-3495(83)84362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A., Sachs F. Single-channel currents from acetylcholine receptors in embryonic chick muscle. Kinetic and conductance properties of gaps within bursts. Biophys J. 1984 Jan;45(1):187–198. doi: 10.1016/S0006-3495(84)84147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F. A high capacity data recording device based on a digital audio processor and a video cassette recorder. Biophys J. 1985 Mar;47(3):437–441. doi: 10.1016/S0006-3495(85)83935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busath D., Szabo G. Gramicidin forms multi-state rectifying channels. Nature. 1981 Nov 26;294(5839):371–373. doi: 10.1038/294371a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Damle V. N., Karlin A. Effects of agonists and antagonists on the reactivity of the binding site disulfide in acetylcholine receptor from Torpedo californica. Biochemistry. 1980 Aug 19;19(17):3924–3932. doi: 10.1021/bi00558a006. [DOI] [PubMed] [Google Scholar]
- Dwyer T. M., Adams D. J., Hille B. The permeability of the endplate channel to organic cations in frog muscle. J Gen Physiol. 1980 May;75(5):469–492. doi: 10.1085/jgp.75.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M., Hall J. E., Mead C. A. The nature of the voltage-dependent conductance induced by alamethicin in black lipid membranes. J Membr Biol. 1973 Dec 31;14(2):143–176. doi: 10.1007/BF01868075. [DOI] [PubMed] [Google Scholar]
- Froehner S. C., Karlin A., Hall Z. W. Affinity alkylation labels two subunits of the reduced acetylcholine receptor from mammalian muscle. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4685–4688. doi: 10.1073/pnas.74.10.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Bormann J., Sakmann B. Activation of multiple-conductance state chloride channels in spinal neurones by glycine and GABA. 1983 Oct 27-Nov 2Nature. 305(5937):805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Sakmann B. Multiple conductance states of single acetylcholine receptor channels in embryonic muscle cells. Nature. 1981 Dec 3;294(5840):462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- Horn R., Lange K. Estimating kinetic constants from single channel data. Biophys J. 1983 Aug;43(2):207–223. doi: 10.1016/S0006-3495(83)84341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. Y., Catterall W. A., Ehrenstein G. Selectivity of cations and nonelectrolytes for acetylcholine-activated channels in cultured muscle cells. J Gen Physiol. 1978 Apr;71(4):397–410. doi: 10.1085/jgp.71.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988 Oct 13;335(6191):645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Jackson M. B. Dependence of acetylcholine receptor channel kinetics on agonist concentration in cultured mouse muscle fibres. J Physiol. 1988 Mar;397:555–583. doi: 10.1113/jphysiol.1988.sp017019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Kinetics of unliganded acetylcholine receptor channel gating. Biophys J. 1986 Mar;49(3):663–672. doi: 10.1016/S0006-3495(86)83693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Lecar H., Askanas V., Engel W. K. Single cholinergic receptor channel currents in cultured human muscle. J Neurosci. 1982 Oct;2(10):1465–1473. doi: 10.1523/JNEUROSCI.02-10-01465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Spontaneous openings of the acetylcholine receptor channel. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3901–3904. doi: 10.1073/pnas.81.12.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Stochastic behavior of a many-channel membrane system. Biophys J. 1985 Feb;47(2 Pt 1):129–137. doi: 10.1016/s0006-3495(85)83886-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao P. N., Dwork A. J., Kaldany R. R., Silver M. L., Wideman J., Stein S., Karlin A. Identification of the alpha subunit half-cystine specifically labeled by an affinity reagent for the acetylcholine receptor binding site. J Biol Chem. 1984 Oct 10;259(19):11662–11665. [PubMed] [Google Scholar]
- Karlin A., Bartels E. Effects of blocking sulfhydryl groups and of reducing disulfide bonds on the acetylcholine-activated permeability system of the electroplax. Biochim Biophys Acta. 1966 Nov 8;126(3):525–535. doi: 10.1016/0926-6585(66)90010-0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A re-examination of curare action at the motor endplate. Proc R Soc Lond B Biol Sci. 1978 Dec 4;203(1151):119–133. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- Manalis R. S. Voltage-dependent effect of curare at the frog neuromuscular junction. Nature. 1977 May 26;267(5609):366–368. doi: 10.1038/267366a0. [DOI] [PubMed] [Google Scholar]
- Marty A., Neild T., Ascher P. Voltage sensitivity of acetylcholine currents in Aplysia neurones in the presence of curare. Nature. 1976 Jun 10;261(5560):501–503. doi: 10.1038/261501a0. [DOI] [PubMed] [Google Scholar]
- Morris C. E., Montpetit M. Multiple conductance states of the acetylcholine receptor channel complex. Can J Physiol Pharmacol. 1986 Mar;64(3):347–355. doi: 10.1139/y86-057. [DOI] [PubMed] [Google Scholar]
- Morris C. E., Wong B. S., Jackson M. B., Lecar H. Single-channel currents activated by curare in cultured embryonic rat muscle. J Neurosci. 1983 Dec;3(12):2525–2531. doi: 10.1523/JNEUROSCI.03-12-02525.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden D. C., Colquhoun D. Ion channel block by acetylcholine, carbachol and suberyldicholine at the frog neuromuscular junction. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):329–355. doi: 10.1098/rspb.1985.0065. [DOI] [PubMed] [Google Scholar]
- Popot J. L., Changeux J. P. Nicotinic receptor of acetylcholine: structure of an oligomeric integral membrane protein. Physiol Rev. 1984 Oct;64(4):1162–1239. doi: 10.1152/physrev.1984.64.4.1162. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Agonists block currents through acetylcholine receptor channels. Biophys J. 1984 Aug;46(2):277–283. doi: 10.1016/S0006-3495(84)84022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Trautmann A. A patch-clamp study of the partial agonist actions of tubocurarine on rat myotubes. J Physiol. 1984 Apr;349:353–374. doi: 10.1113/jphysiol.1984.sp015160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank D. W., Huganir R. L., Greengard P., Webb W. W. Patch-recorded single-channel currents of the purified and reconstituted Torpedo acetylcholine receptor. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5129–5133. doi: 10.1073/pnas.80.16.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. W., Lukas R. J., McNamee M. G. Effects of thio-group modifications on the ion permeability control and ligand binding properties of Torpedo californica acetylcholine receptor. Biochemistry. 1981 Apr 14;20(8):2191–2199. doi: 10.1021/bi00511a018. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind L., Dennis M. J. Depolarising effect of curare on embryonic rat muscles. Nature. 1978 Dec 7;276(5688):622–623. doi: 10.1038/276622a0. [DOI] [PubMed] [Google Scholar]
- de Souza Otero A., Hamilton S. L. Ligand-induced variations in the reactivity of thio groups of the alpha-subunit of the acetylcholine receptor from Torpedo californica. Biochemistry. 1984 May 22;23(11):2321–2328. doi: 10.1021/bi00306a001. [DOI] [PubMed] [Google Scholar]


