Abstract
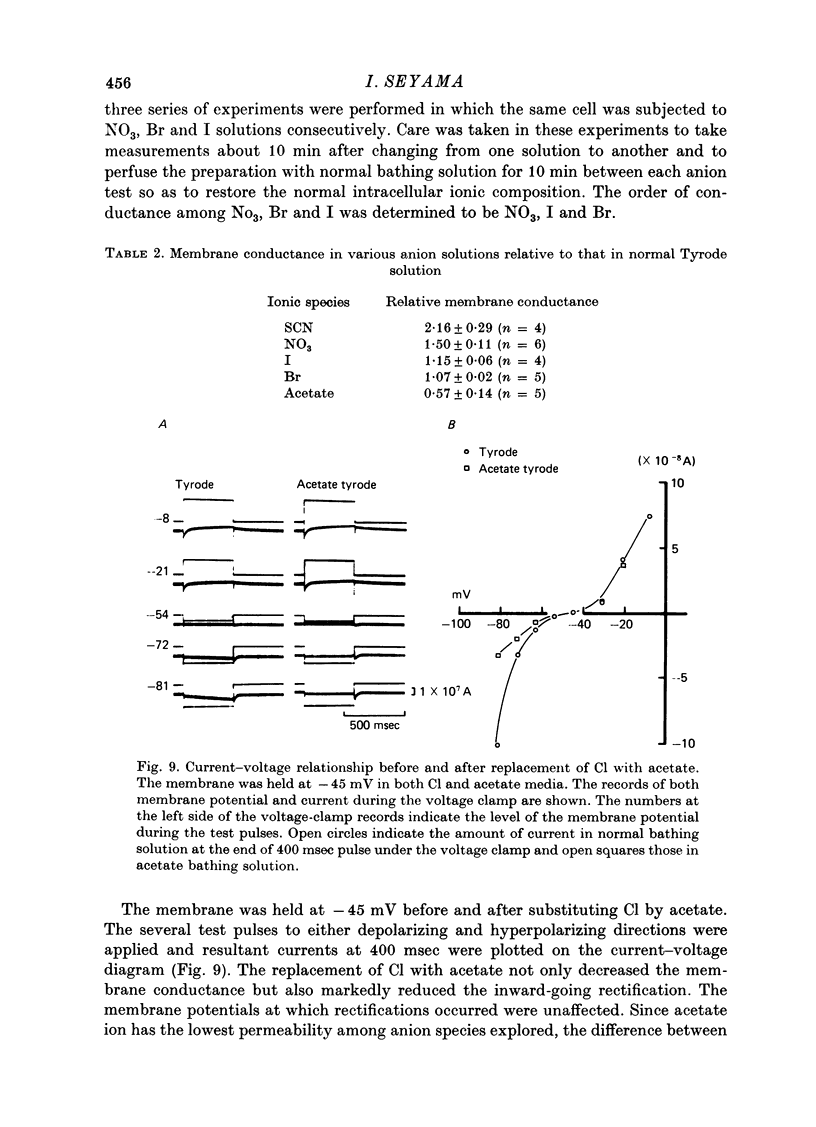
1. The anion permeability of the sino-atrial node cell membranes was determined by substituting various anions for Cl and observing the resultant transient changes in membrane potential. The permeability sequence was found to be in the following order: thiocyanate greater than NO3 greater than I Br greater than Cl greater than acetate. 2. The membrane resistance in Cl solution was compared with that in various anion solutions by the voltage-clamp method. The conductance sequence for the sino-atrial node cell membrane was observed to be the same as the permeability sequence. 3 The potential generated by the Na-K pump was partly short-circuited in normal bathing solution, and thus the pump could be responsible in part for the generation of the sin-atrial node resting membrane potential. 4 When Cl was replaced by acetate, a less permeable ion, the inward-going rectification disappeared. Thus, the inward-going rectification might be due partly to a time- and voltage-dependent Cl current.
Full text
PDF


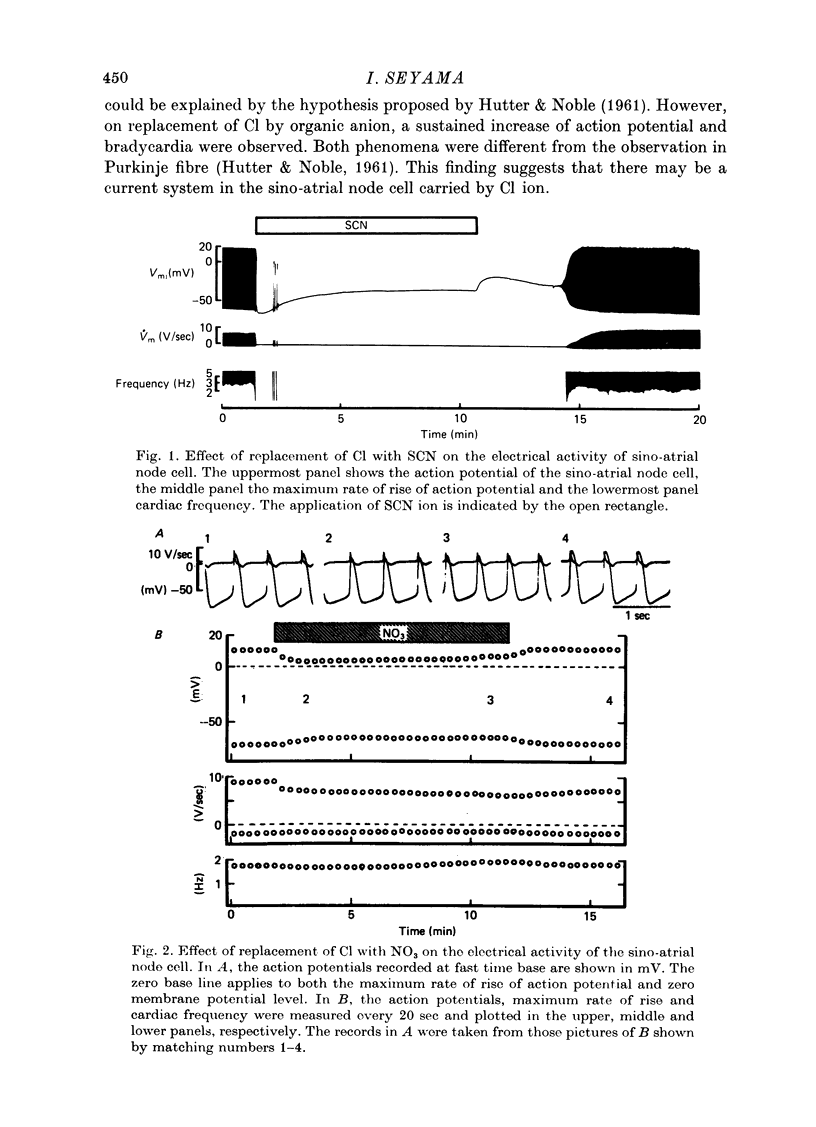
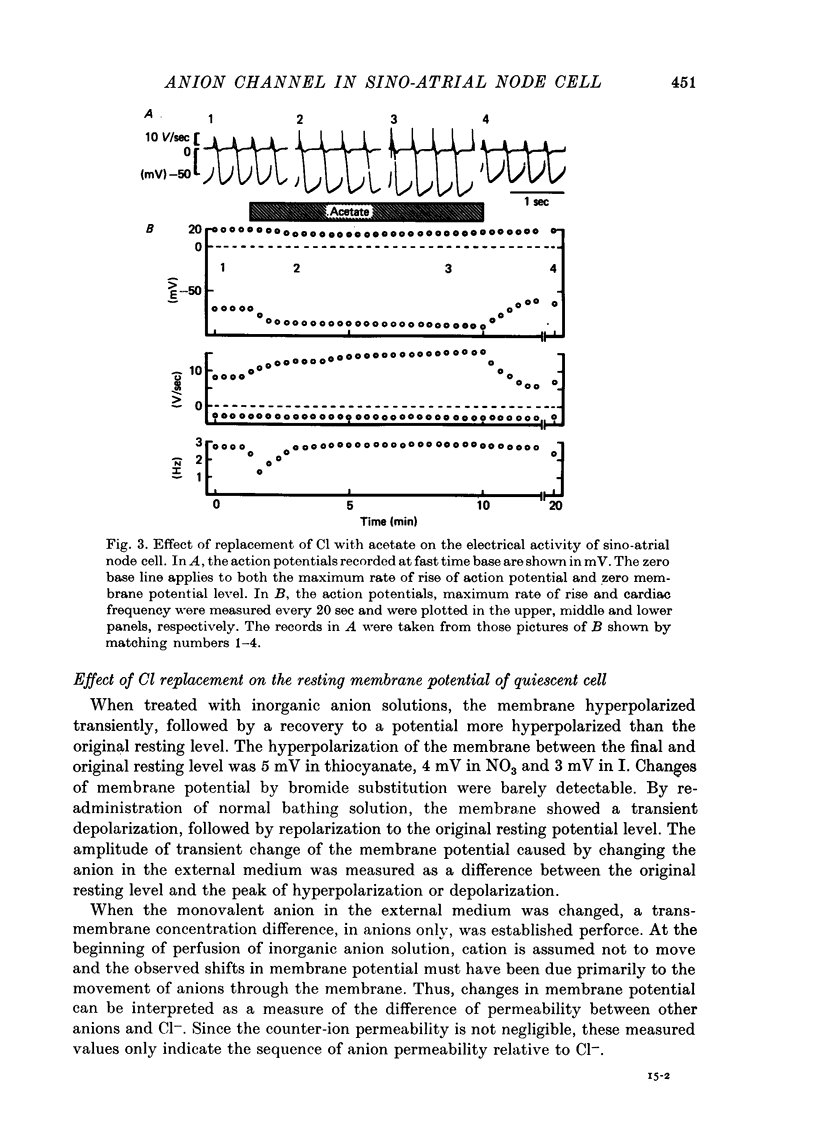
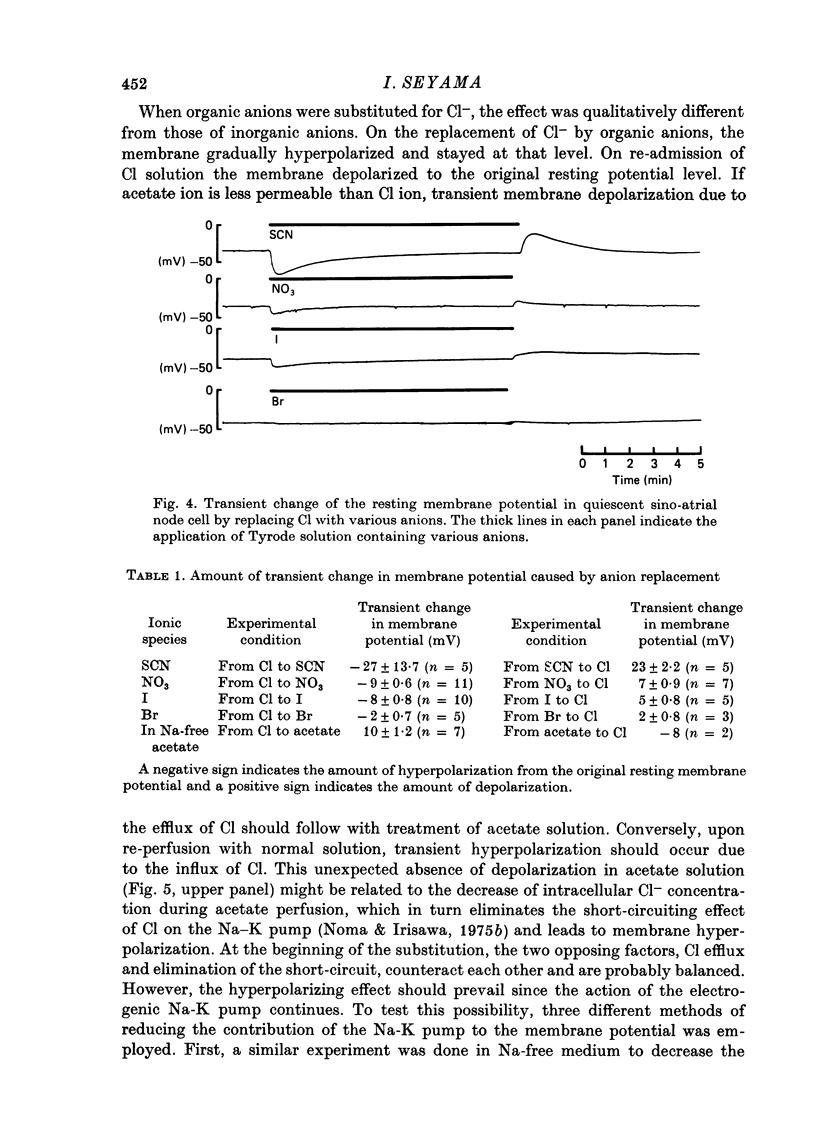
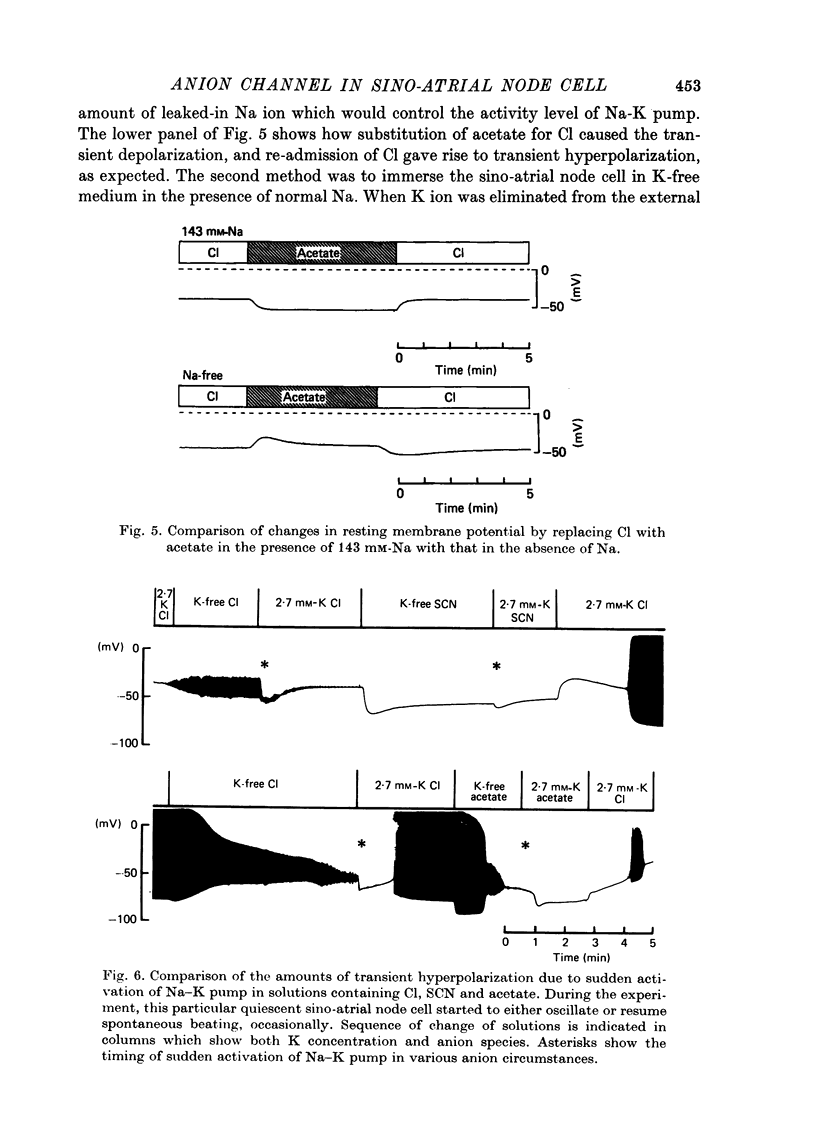






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton T. B., Vaughan-Jones R. D. Continuous direct measurement of intracellular chloride and pH in frog skeletal muscle. J Physiol. 1977 Sep;270(3):801–833. doi: 10.1113/jphysiol.1977.sp011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., Giles W., Noble S. J. Membrane currents underlying activity in frog sinus venosus. J Physiol. 1977 Oct;271(3):783–816. doi: 10.1113/jphysiol.1977.sp012026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARMELIET E. E. Chloride ions and the membrane potential of Purkinje fibres. J Physiol. 1961 Apr;156:375–388. doi: 10.1113/jphysiol.1961.sp006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E., Verdonck F. Reduction of potassium permeability by chloride substitution in cardiac cells. J Physiol. 1977 Feb;265(1):193–206. doi: 10.1113/jphysiol.1977.sp011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. The distribution of chloride ions in the smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1971 Apr;214(2):225–243. doi: 10.1113/jphysiol.1971.sp009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMELLO W. C. ROLE OF CHLORIDE IONS IN CARDIAC ACTION AND PACEMAKER POTENTIALS. Am J Physiol. 1963 Sep;205:567–575. doi: 10.1152/ajplegacy.1963.205.3.567. [DOI] [PubMed] [Google Scholar]
- Diamond J. M., Wright E. M. Biological membranes: the physical basis of ion and nonelectrolyte selectivity. Annu Rev Physiol. 1969;31:581–646. doi: 10.1146/annurev.ph.31.030169.003053. [DOI] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. Anion conductance of cardiac muscle. J Physiol. 1961 Jul;157:335–350. doi: 10.1113/jphysiol.1961.sp006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Toyama K., Hayashi H. Mechanisms of anion and cation permeations in the resting membrane of a barnacle muscle fiber. J Gen Physiol. 1971 Apr;57(4):408–434. doi: 10.1085/jgp.57.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. Effects of low-chloride solutions on action potentials of sheep cardiac Purkinje fibers. J Gen Physiol. 1977 Nov;70(5):635–660. doi: 10.1085/jgp.70.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladle R. O., Walker J. L. Intracellular chloride activity in frog heart. J Physiol. 1975 Oct;251(2):549–559. doi: 10.1113/jphysiol.1975.sp011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A., Irisawa H. A time- and voltage-dependent potassium current in the rabbit sinoatrial node cell. Pflugers Arch. 1976 Nov 5;366(2-3):251–258. doi: 10.1007/BF00585886. [DOI] [PubMed] [Google Scholar]
- Noma A., Irisawa H. Contribution of an electrogenic sodium pump to the membrane potential in rabbit sinoatrial node cells. Pflugers Arch. 1975 Aug 12;358(4):289–301. doi: 10.1007/BF00580527. [DOI] [PubMed] [Google Scholar]
- Noma A., Irisawa H. Effects of Na+ and K+ on the resting membrane potential of the rabbit sinoatrial node cell. Jpn J Physiol. 1975;25(3):207–302. [PubMed] [Google Scholar]
- Russell J. M. ATP-Dependent chloride influx into internally dialyzed squid giant axons. J Membr Biol. 1976 Sep 17;28(4):335–349. doi: 10.1007/BF01869704. [DOI] [PubMed] [Google Scholar]
- Seyama I. Characteristics of the rectifying properties of the sino-atrial node cell of the rabbit. J Physiol. 1976 Feb;255(2):379–397. doi: 10.1113/jphysiol.1976.sp011285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyama I. The effect of Na, K and Cl ions on the resting membrane potential of sino-atrial node cell of the rabbit. Jpn J Physiol. 1977;27(5):577–588. doi: 10.2170/jjphysiol.27.577. [DOI] [PubMed] [Google Scholar]
- Wang C. M., Narahashi T., Scuka M. Mechanism of negative temperature coefficient of nerve blocking action of allethrin. J Pharmacol Exp Ther. 1972 Sep;182(3):442–453. [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Anion selectivity in biological systems. Physiol Rev. 1977 Jan;57(1):109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]


