Abstract
Hemozoin (malaria pigment) has been implicated in the modulation of immune responses during malaria infection. This study was designed to evaluate the effect of purified hemozoin on the in vitro activation of myeloid dendritic cells. Our study also revealed that in addition to enhancing the maturation of dendritic cells, hemozoin also greatly promotes immunoglobulin G2a antibody responses when coadministered with a DNA vaccine plasmid encoding Pfs25, a Plasmodium falciparum transmission-blocking antigen.
Malaria-associated immunosuppression has been widely investigated, and it has been suggested that malaria pigment, also known as hemozoin (HZ), may participate in the mechanisms underlying it (1, 8, 10, 15, 16, 17). Plasmodium parasites detoxify heme molecules in the food vacuole into HZ (13), which persists inside the parasite. During schizont rupture, intracellular HZ released into the circulation is subsequently concentrated in the reticuloendothelial system of the host, where it may persist unchanged in macrophages for several months (16). In vivo experiments have shown that during malaria infection, HZ loading severely impairs the function of phagocytes. HZ-loaded monocytes are impaired in the generation of oxidative burst, in the ability to repeat phagocytosis, and in protein kinase C activity (1, 14, 15). Other studies have also revealed that phagocytosis of opsonized HZ impairs the expression of major histocompatibility complex class II antigen, CD54, and CD11c in human monocytes (16).
Recently, it was found that dendritic cell (DC) maturation was severely inhibited by intact Plasmodium falciparum-infected erythrocytes (26). DCs, as crucial antigen-presenting cells, are more effective in activating naïve T cells and thus in initiating adaptive immune responses. They also activate innate immune responses to microbial challenge by producing cytokines such as interleukin-12 (IL-12) and interferons (5, 9, 21). Although the role of HZ when phagocytosed by human monocytes has been examined extensively, the interaction between HZ and DCs has not been examined. DCs are also important for the immunogenicity of DNA vaccine-encoded antigens. They control the interaction between transfected somatic cells and T cells, as well as the delivery of antigen to secondary lymphoid organs (2, 18, 23). The goal of the present investigation was to assess the direct immunomodulatory effect of purified HZ on DC maturation and cytokine production as well as the effect of HZ on DNA vaccine-induced antibody responses.
Since previous studies employed rather crude HZ preparations and parasite lysates, it was difficult to distinguish between the role of HZ and those of contaminating proteins, membranes, and glycosylphosphatidylinositol molecules (11), etc. In the present study, the HZ purification procedure that was employed eliminated all of the above-mentioned copurifying molecules. We also carefully examined the endotoxin levels of purified HZ because human peripheral blood mononuclear cells are extremely sensitive to even trace amounts of lipopolysaccharide (LPS) (19). In contrast to previous findings on the immunosuppressive effects of crude HZ preparations on monocytes and macrophages, we found that purified HZ upregulates DC maturation, as revealed by marked increases in both cell surface molecules and in IL-12 production in DCs.
HZ was purified from P. falciparum (strain 3D7)-infected erythrocytes (25). When the level of parasites, mostly trophozoites, reached more than 10% in the culture, parasites were harvested by saponin lysis as described before (3). Briefly, saponin-lysed parasites were washed three to five times with phosphate-buffered saline (PBS) and sonicated in 2% sodium dodecyl sulfate (SDS). Following seven to eight washes in 2% SDS, the pellet was resuspended in a solution of 10 mM Tris-HCl (pH 8.0), 0.5% SDS, and 1 mM CaCl2 containing 2 mg of proteinase K per ml and was then incubated at 37°C overnight. The pellet was then washed three times in 2% SDS and incubated in 6 M urea for 3 h at room temperature on a shaker. Following three to five washes in 2% SDS and then in distilled water, the HZ pellet was resuspended in distilled water and sonicated again prior to use to minimize aggregation and maintain the HZ in suspension. Previous studies (20) have established the purity of HZ prepared by the method described above. In these experiments, we also used heme (heme monomer) and synthetic β-hematin as a control to HZ. β-Hematin was prepared from heme by using an acetic acid treatment described previously (3,4). Heme stock (600 μM) was prepared by dissolving 40 mg of hemin (Sigma) in 300 μl of 1 M NaOH (24). The pH was adjusted to 7.5 by the addition of 1 M HEPES, and the final volume was adjusted to 100 ml with RPMI medium. The concentrations of all solutions were determined by depolymerizing the heme polymers in 1 ml of a 20 mM sodium hydroxide-2% SDS solution for 2 h at room temperature and by measuring the absorbance at 400 nm. The molar extinction coefficient for heme is 105 at 400 nm (22). Initial experiments on DC maturation and cytokine production were done at various concentrations (1, 3, and 10 μM) of various test preparations and revealed a dose-dependent response. We chose to use a 10 μM concentration so that we could observe the direct effect of HZ without any interference from any unknown toxicity or trace amount of LPS. Since monocytes and DCs are extremely sensitive to LPS contaminations, we also employed various solutions prepared in endotoxin-free PBS and water. Endotoxin levels measured by Limulus amoebocyte lysate assays (BioWhittaker, Walkersville, Md.) were below 0.0125 endotoxin unit for each nanomole of HZ used.
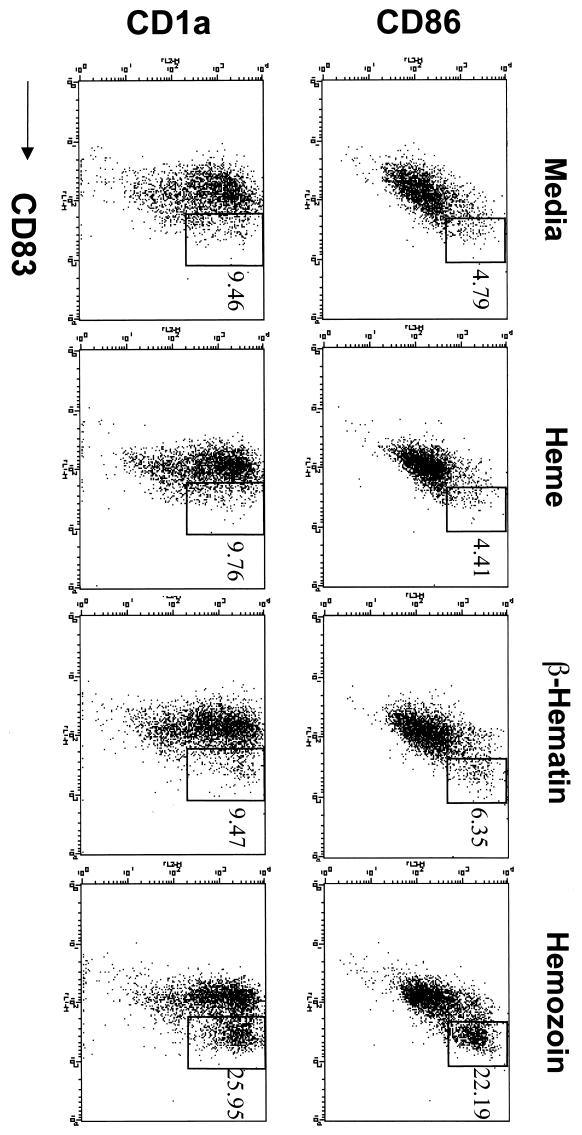
Immature human myeloid DCs were obtained from elutriated monocytes that had been cultured for 7 days with human granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D Systems, Minneapolis, Minn.) (100 ng/ml) and human IL-4 (R&D Systems) (25 ng/ml) in RPMI medium containing 10% fetal bovine serum (12). Immature DCs were then stimulated further with 10 μM purified HZ for 36 h at 37°C. Fluorescence-activated cell sorter (FACS) analysis was carried out using fluorescein isothiocyanate-, phycoerythrin-, and/or CyChrome-labeled antibodies against human CD1a, CD83, and CD86 (BD PharMingen, San Diego, Calif.) as recommended by the manufacturer. Cells (104) were analyzed by FACSort (BD Biosciences), and Cell Quest software (BD Biosciences) was used for data analysis. FACS analysis showed that purified HZ upregulated the surface molecules CD83, CD86, and CD1a, which are maturation markers for DCs (Fig. 1). Purified heme or synthetic β-hematin, on the other hand, did not alter CD83, CD86, or CD1a surface molecules. These experiments were repeated with two independent preparations of purified HZ, and results showed that P. falciparum consistently enhanced in vitro maturation of immature DCs. We also incubated elutriated monocytes with 10 μM heme, β-hematin, and HZ for 48 h and did not detect any upregulation of DC maturation surface markers (CD86, CD83, or CD1a), further suggesting a direct role for HZ during the in vitro maturation of immature DCs (data not shown).
FIG. 1.
Hemozoin upregulates DC surface markers. Elutriated human monocytes were incubated with GM-CSF and IL-4, and immature DCs were further incubated with 10 μM HZ, β-hematin, or heme for 36 h. Upregulation of CD83, CD86, and CD1a surface markers was measured by FACS analysis. The data shown are representative of two experiments repeated with two independent preparations of highly purified HZ.
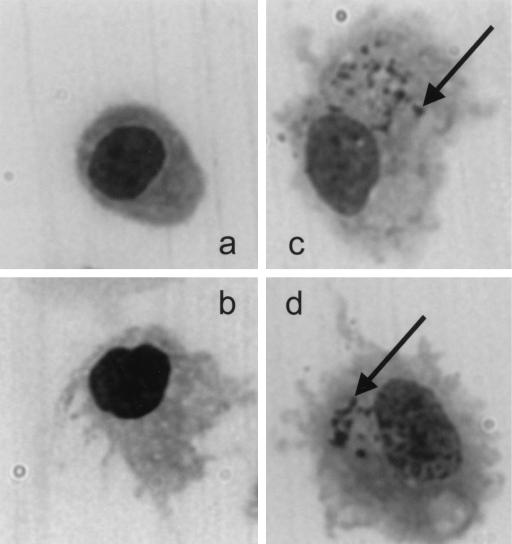
HZ-mediated enhanced DC maturation, as suggested by the upregulation of surface markers, was also confirmed by morphological changes in the cells. Immature DCs were incubated with HZ, LPS, and HZ plus LPS for 48 h, and slides (cytospin) were fixed with ethanol. After the slides were stained with Giemsa stain for 20 min, bright-field microscopy (magnification, ×600; Olympus America, Melville, N.Y.) was used for analysis. Figure 2 shows the mature DCs after the internalization of HZ. The activation of DC maturation by HZ is thus similar to that of proinflammatory cytokines (tumor necrosis factor alpha and IL-1β) and bacterial and viral products such as LPS, immunostimulatory CpG-containing oligodeoxynucleotides, and double-stranded RNA (5, 9, 21).
FIG. 2.
DC morphology after incubation with hemozoin. Immature DCs (a) were stimulated with LPS as a positive control (b), with HZ only (c), or with HZ plus LPS (d) for 48 h. Giemsa-stained cytospin preparations were examined at ×600 magnification. Arrows indicate the HZ internalized by DCs.
DCs and macrophages are the major source of IL-12 (5). During hematopoiesis, monocytes, as a subset of DC precursors, differentiate into immature myeloid DCs in culture with GM-CSF and IL-4 (6) and secrete IL-12 during their further maturation. Immature DCs obtained from elutriated monocytes by culture in the presence of GM-CSF and IL-4 for 7 days were incubated with HZ for 36 h; supernatant was then examined for IL-12. Immulon 2 plates were coated with 5 μg of anti-human IL-12 per ml and blocked with 1% bovine serum albumin-PBS. Culture supernatants were tested in triplicate. Following incubation with biotinylated anti-cytokine antibody (1 μg/ml) and phosphatase-streptavidin (BD PharMingen), the plates were developed using K-gold substrate (Neogen, Lexington, Ky.) and absorbance was read at 405 nm. Figure 3 shows results for DCs from two different donors. The presence of HZ during LPS stimulation did not alter the LPS-induced IL-12 response (data not shown).
FIG. 3.
Hemozoin induces DC to produce IL-12. After incubation of DCs with HZ, IL-12 levels were detected in the supernatants by using a cytokine ELISA. Results (averages + standard errors for triplicate cultures) of two different experiments with DCs from two different donors are shown.
Since DCs are also important for in vivo immune responses to antigens, we were interested in testing the effect of HZ on DNA vaccine-induced humoral immune responses against Pfs25, a transmission-blocking vaccine antigen (7). Four- to six-week-old female BALB/c mice (four mice per group) were immunized intramuscularly with 25 μg of DNA vaccine (VR1020-Pfs25 encoding Pfs25) in the presence of 1 or 20 nmol of HZ. Four weeks after this primary immunization, the mice were given a booster of 25 μg of DNA vaccine (VR1020-Pfs25) alone. After another 4 weeks, antibody titers and antibody isotypes were measured by an enzyme-linked immunosorbent assay (ELISA) as described before (7). Although DNA immunization with HZ did not affect antigen-specific total IgG titers, the isotypes of specific antibodies were significantly different (Fig 4). The ratio of IgG2a to IgG1 antibodies was significantly higher in the group injected with 20 nmol of HZ plus DNA than in the group that received DNA vaccine alone (P < 0.03).
FIG. 4.
Effects of hemozoin on DNA vaccine-induced antibody isotypes. Antibody titers to rPfs25 were measured by ELISA. Four BALB/c mice for each group were analyzed individually, and the IgG2a/IgG1 ratio was calculated for each mouse. Averages + standard deviations are shown for each group. Results are representative of two different animal experiments at different times. Statistical significance (∗) was determined by Student's t test (P < 0.03).
The primary goal of these studies was to evaluate a direct role for HZ in the immunomodulation during malaria infection and its effect on DNA vaccine-induced humoral immune responses. Urban et al. showed that malaria-infected erythrocytes adhere to DCs, inhibit the maturation of DCs into fully competent antigen-presenting cells, and reduce their capacity to stimulate T cells (26). In the same study, they also observed that a crude pigment preparation derived from infected erythrocytes did not have any effect on the mature DCs induced by LPS. More recent studies have shown that DC modulation in vitro by infected red blood cells is a contact-dependent process which requires critical levels of infected red blood cells and which depends on the infected red blood cells' affinity for CD36 (27). While this modulation of DC may result in impaired antiparasite immune responses, our studies demonstrate that the internalization of parasite products like HZ may actually activate DCs. These studies thus raise the possibility that other parasite components may downregulate the immune response during malaria infection. The effect of HZ, when administered during primary immunization with DNA vaccine, on antibody isotype switching also warrants further investigation as a potential immunomodulator.
Acknowledgments
We thank the NIH Blood Bank for supplying elutriated monocytes. We also thank Mayda Gursel for critical comments as well as technical support.
These studies were supported partially by NIH grant AI 47089.
C. Coban and K. J. Ishii contributed equally to this work.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Arese, P., and E. Schwarzer. 1997. Malarial pigment (haemozoin): a very active ‘inert’ substance. Ann. Trop. Med. Parasitol. 91:501-516. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 3.Chen, M. M., L. Shi, and D. J. Sullivan, Jr. 2001. Haemoproteus and Schistosoma synthesize heme polymers similar to Plasmodium hemozoin and β-hematin. Mol. Biochem. Parasitol. 113:1-8. [DOI] [PubMed] [Google Scholar]
- 4.Egan, T., D. Ross, and P. Adams. 1994. Quinoline anti-malarial drugs inhibit spontaneous formation of β-haematin (malaria pigment). FEBS Lett. 352:54-61. [DOI] [PubMed] [Google Scholar]
- 5.Lanzavecchia, A. 1999. Dendritic cell maturation and generation of immune response. Haematologica 84:23-25. [PubMed] [Google Scholar]
- 6.Liu, Y. 2001. Dendritic cell subsets and lineages, and their functions in innate and adaptive responses. Cell 106:259-262. [DOI] [PubMed] [Google Scholar]
- 7.Lobo, C. A., R. Dhar, and N. Kumar. 1999. Immunization of mice with DNA-based Pfs25 elicits potent malaria transmission-blocking antibodies. Infect. Immun. 67:1688-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luty, A. J. F., D. J. Perkins, B. Lell, R. Schmidt-Ott, L. G. Lehman, D. Luckner, B. Greve, P. Matousek, K. Herbich, D. Schmid, J. B. Weinberg, and P. G. Kremsner. 2000. Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect. Immun. 68:3909-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mellman, I., and R. M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255-258. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen, P. H., N. Day, T. D. Pram, D. J. Ferguson, and N. J. White. 1995. Intraleukocytic malaria pigment and prognosis in severe malaria. Trans. R. Soc. Trop. Med. Hyg. 89:200-204. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandra, S. N., O. H. Branch, A. S. Woods, M. Vijaykumar, D. J. Perkins, B. L. Nahlen, A. A. Lal, R. J. Cotter, C. E. Costello, C. F. Ockenhouse, E. A. Davidson, and D. C. Gowda. 2000. Glycosylphosphatidylinositol anchors of P. falciparum: molecular characterization and naturally elicited antibody response that may provide immunity to malaria pathogenesis. J. Exp. Med. 192:1563-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romani, N., S. Gruner, D. Brang, E. Kampgen, A. Lenz, B. Trockenbacher, G. Konwalinka, P. O. Fritsch, R. M. Steinman, and G. Schuler. 1994. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 180:83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenthal, P. J., and S. R. Meshnick. 1998. Hemoglobin processing and the metabolism of amino acids, heme, and iron, p. 145-159. In I. W. Sherman (ed.), Malaria parasite biology, pathogenesis, and protection. American Society for Microbiology, Washington, D.C.
- 14.Schwarzer, E., F. Turrini, G. Giribaldi, M. Cappadoro, and P. Arese. 1993. Phagocytosis of P. falciparum malarial pigment hemozoin by human monocytes inactivates monocyte protein kinase C. Biochim. Biophys. Acta 1181:51-54. [DOI] [PubMed] [Google Scholar]
- 15.Schwarzer, E., F. Turrini, D. Ulliers, G. Giribaldi, H. Ginsburg, and P. Arese. 1992. Impairment of macrophage functions after ingestion of Plasmodium falciparum infected erythrocytes or isolated malarial pigment. J. Exp. Med. 176:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarzer, E., M. Alessio, D. Ulliers, and P. Arese. 1998. Phagocytosis of the malarial pigment, hemozoin, impairs expression of major histocompatibility complex class II antigen, CD54, and CD11c in human monocytes. Infect. Immun. 66:1601-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scorza, T., S. Magez, L. Brys, and P. Baetselier. 1999. Hemozoin is a key factor in the induction of malaria-associated immunosuppression. Parasite Immunol. 21:545-554. [DOI] [PubMed] [Google Scholar]
- 18.Shedlock, D. J., and D. B. Weiner. 2000. DNA vaccination: antigen presentation and the induction of immunity. J. Leukoc. Biol. 68:793-806. [PubMed] [Google Scholar]
- 19.Shoda, L. K. M., K. A. Kegerreis, C. E. Suarez, W. Mwangi, D. P. Knowles, and W. C. Brown. 2001. Immunostimulatory CpG-modified plasmid DNA enhances IL- 12, TNF-α, and NO production by bovine macrophages. J. Leukoc. Biol. 70:103-112. [PubMed] [Google Scholar]
- 20.Slater, A. F. G., W. J. Swiggard, B. R. Orton, W. D. Flitter, D. E. Goldberg, A. Cerami, and G. B. Henderson. 1991. An iron-carboxylase bond links the heme units of malaria pigment. Proc. Natl. Acad. Sci. USA 88:325-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sousa, C. R. 2001. Dendritic cells as sensors of infection. Immunity 14:495-498. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan, D. J., Jr., I. Y. Gluzman, D. G. Russell, and D. E. Goldberg. 1996. On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. USA 93:11865-11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takashima, A., and A. Morita. 1999. Dendritic cells in genetic immunization. J. Leukoc. Biol. 66:350-356. [DOI] [PubMed] [Google Scholar]
- 24.Theodorakis, N. G., D. J. Zand, P. T. Kotzbauer, G. T. Williams, and R. I. Morimoto. 1989. Hemin-induced transcriptional activation of the Hsp70 gene during erythroid maturation in K562 cells is due to a heat shock factor-mediated stress response. Mol. Cell. Biol. 9:3166-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 26.Urban, B. C., D. J. P. Ferguson, A. Pain, N. Willcox, M. Plebanski, J. M. Austin, and D. J. Roberts. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400:73-77. [DOI] [PubMed] [Google Scholar]
- 27.Urban, B. C., N. Willcox, and D. J. Roberts. 2001. A role for CD36 in the regulation of dendritic cell function. Proc. Natl. Acad. Sci. USA 98:8750-8755. [DOI] [PMC free article] [PubMed] [Google Scholar]