Abstract
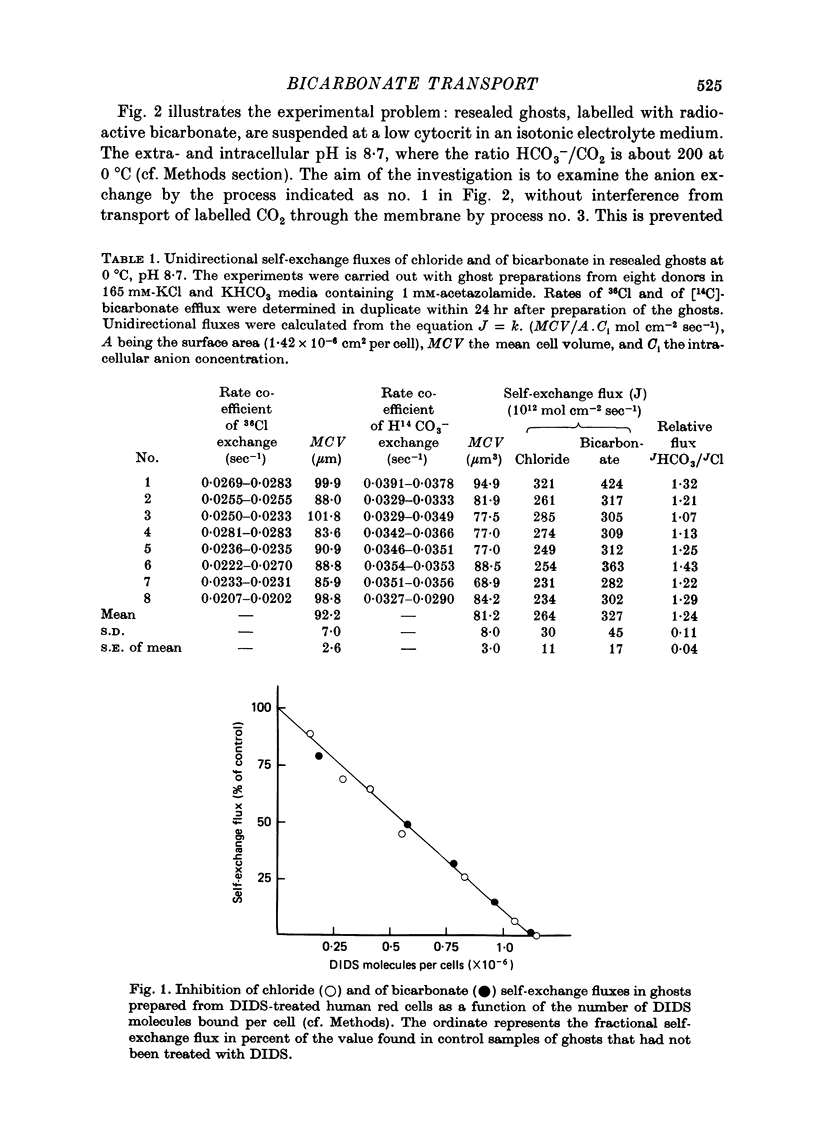
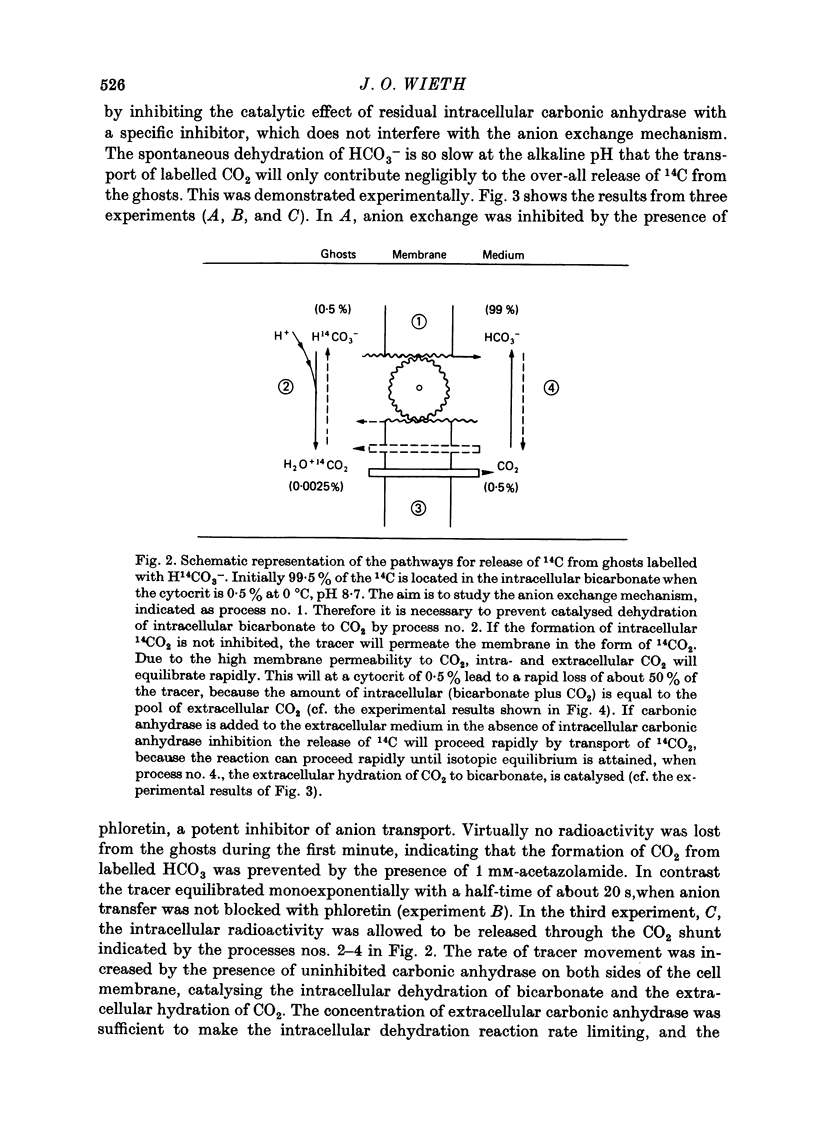
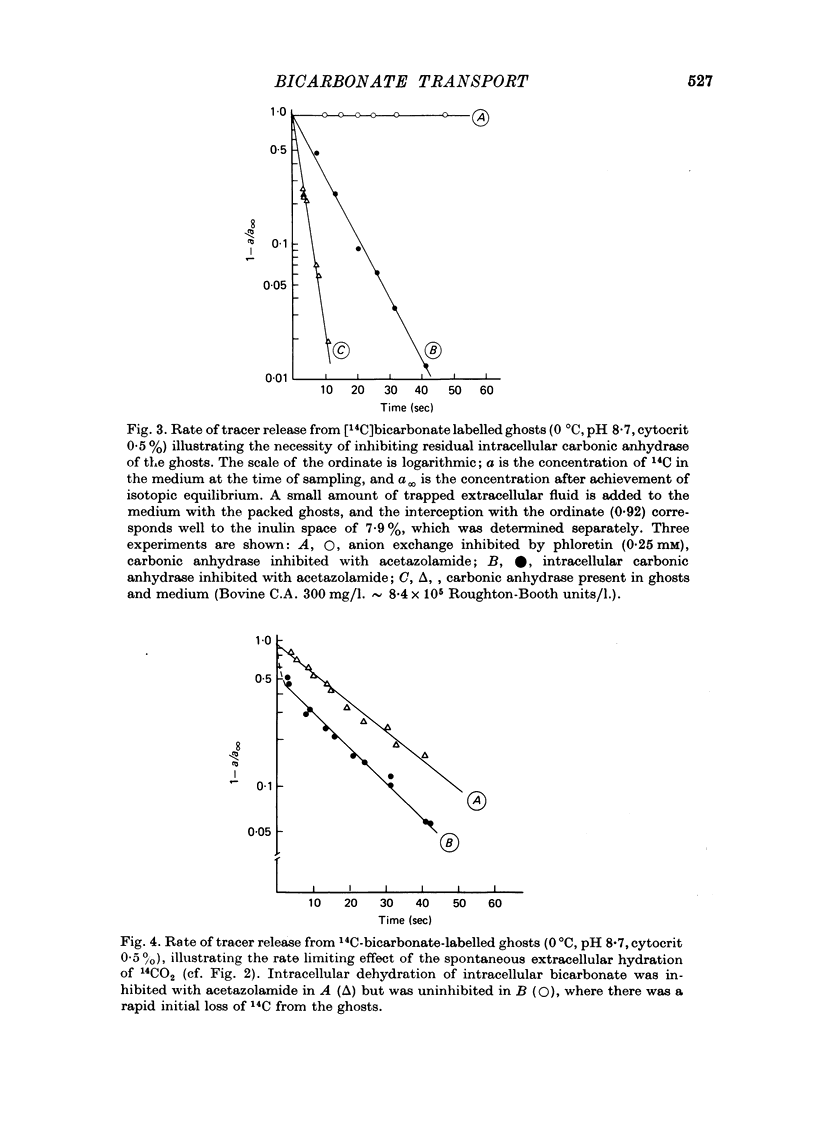
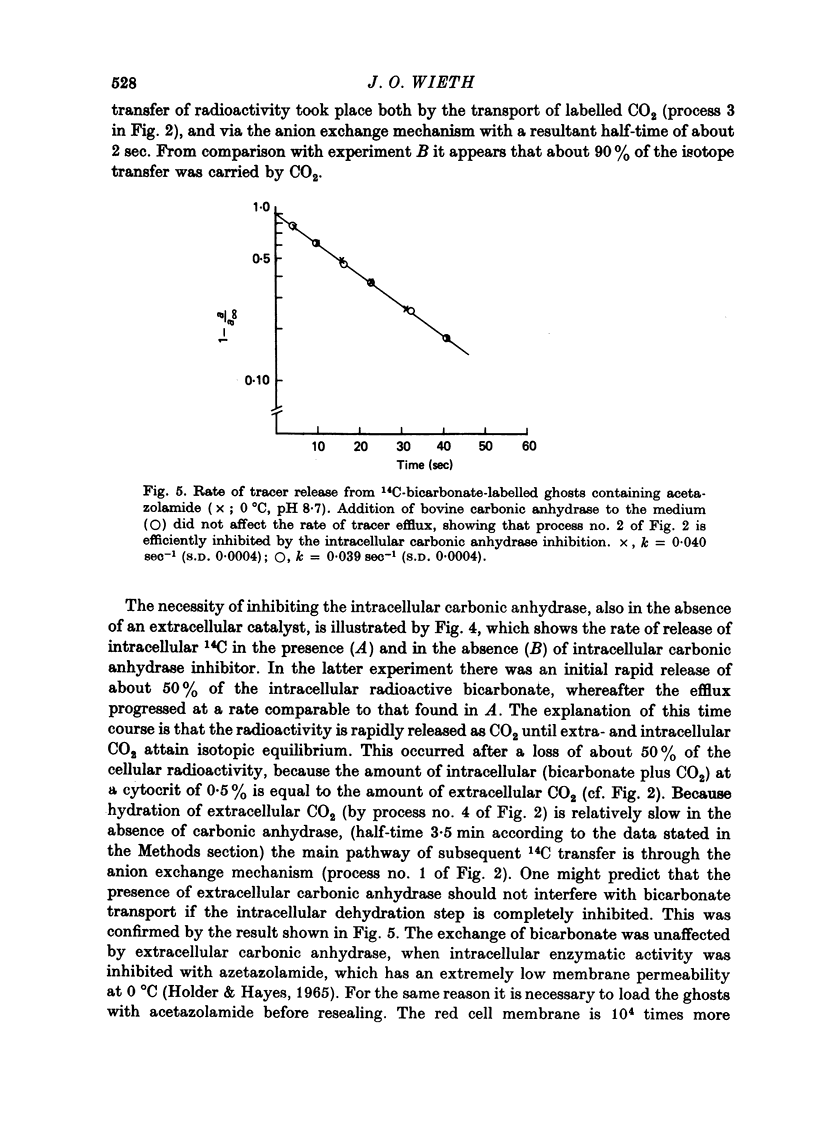
1. Bicarbonate transport across human red cell membranes was studied between 0 and 10 degrees C at alkaline pH values by determining the efflux of 14C-labelled bicarbonate from resealed erythrocyte ghosts. Transfer of labelled CO2 was eliminated as a source of error, when formation of intracellular 14CO2 was inhibited with carbonic anhydrase inhibitors. The study showed that there are no fundamental differences between the characteristics of bicarbonate and of chloride self-exchange as has been inferred from previous studies of chloride-bicarbonate exchange. 2. Efflux of radioactivity could be reduced more than 99% by reversible and irreversible inhibitors of anion transport. Inhibition of both chloride and bicarbonate self-exchange was linearly related to the binding of 4,4'-diisothiocyanostilbene-2,2'-disulphonic acid (DIDS) to the membranes. Complete (i.e. greater than 99%) inhibition was obtained after binding of 1.2 x 10(6) DIDS molecules per cell. 3. Bicarbonate self-exchange proved a saturable function of bicarbonate concentration, with a maximum at external and internal concentrations of approximately 100 mM, showing self-depression at higher bicarbonate concentrations, and half-maximum exchange flux at a concentration of 10 mM. The results were consistent with the hypothesis that the exchange mechanism has two anion binding sites, one mediating ion transport and the other causing transport inhibition. 4. Maximum exchange flux of bicarbonate was about 30% larger thant that of chloride, and the affinity of bicarbonate for the transport site was about three times larger than that of chloride. The apparent activation energy of bicarbonate exchange was 28 kcal/mole, the same order of magnitude as found for other inorganic anions between 0 and 10 degrees C. 5. The ability of other inorganic anions to exchange with bicarbonate decreased in the sequence Cl greater than NO3 greater than F greater than Br greater than or equal to I, corresponding to the sequence of the rate of self-exchange of halides. 6. Counter-transport of bicarbonate could be driven by a chloride gradient, when ghosts containing KCl were suspended in a medium containing traces of labelled bicarbonate in addition to a non-permeating anion. Concentration ratios (ci/co) up to about 1000 could be obtained. 7. It is concluded that bicarbonate is transported by the inorganic anion exchange mechanism of the erythrocyte membrane. The slight differences between the exchange kinetics of chloride and bicarbonate were explained by differing affinities of the two anions for the two anion binding sites of the transport system.
Full text
PDF



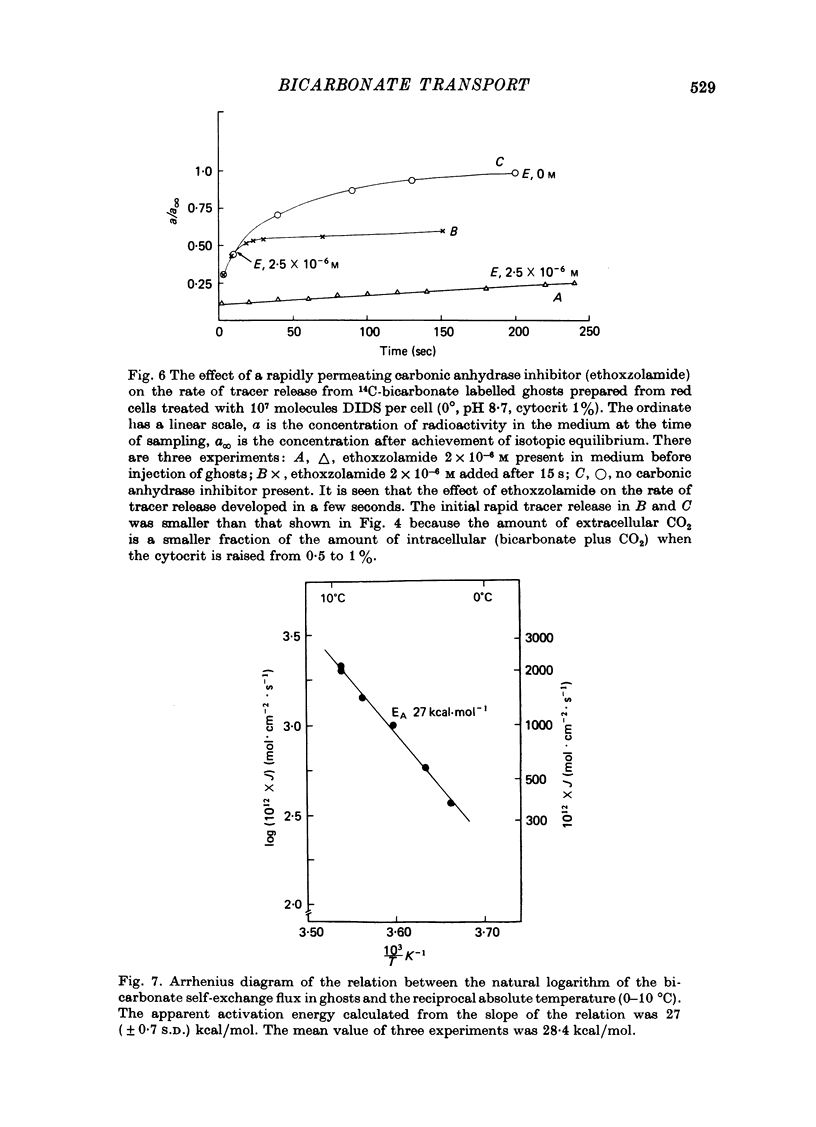
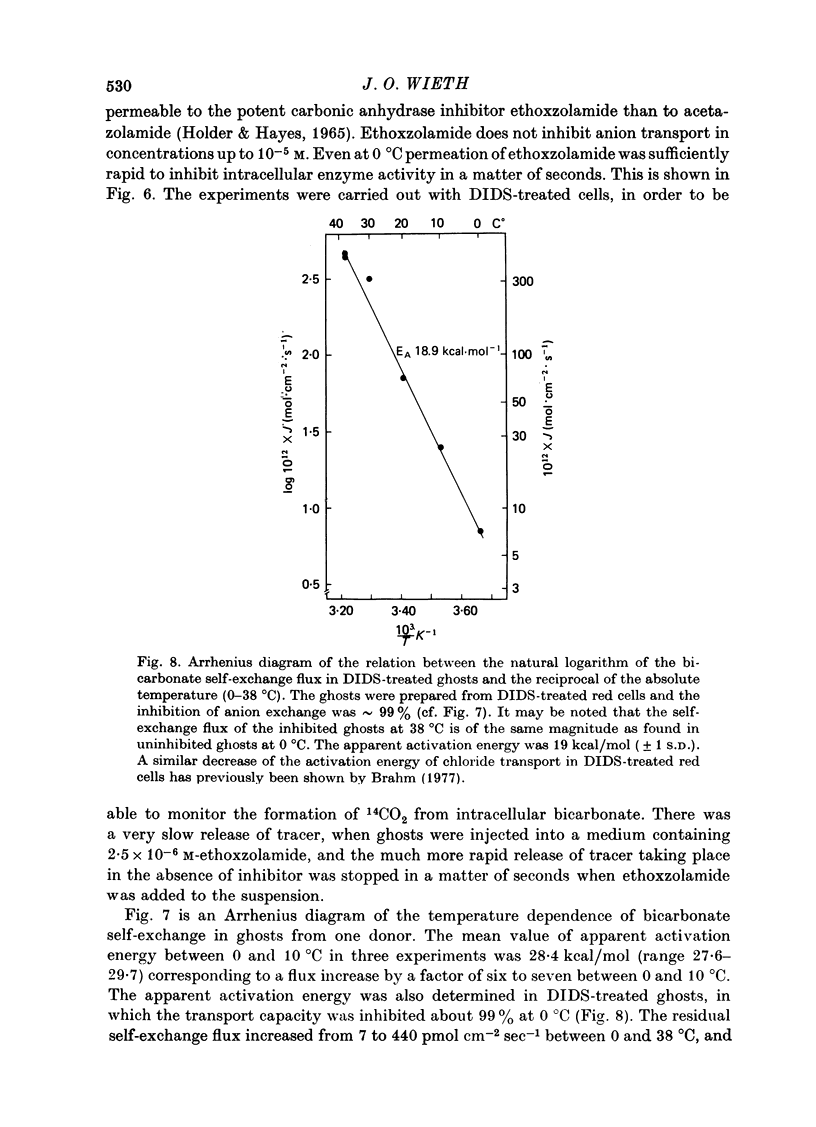
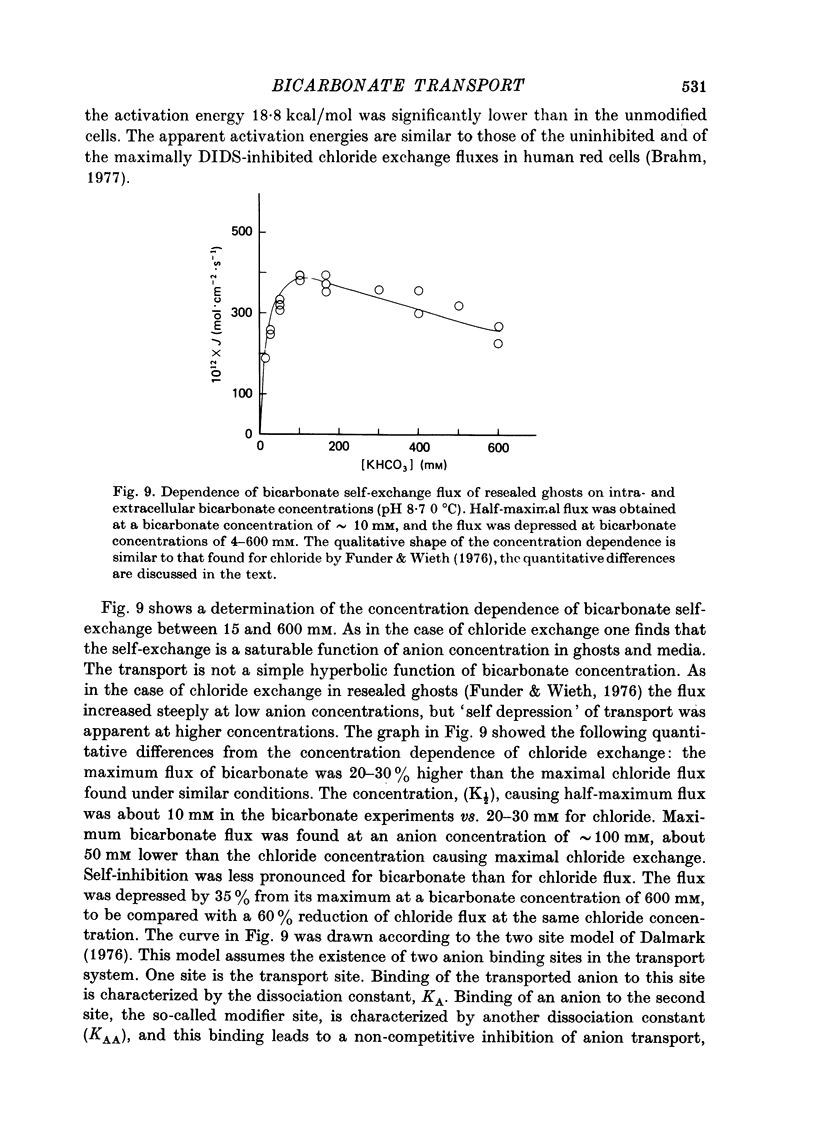
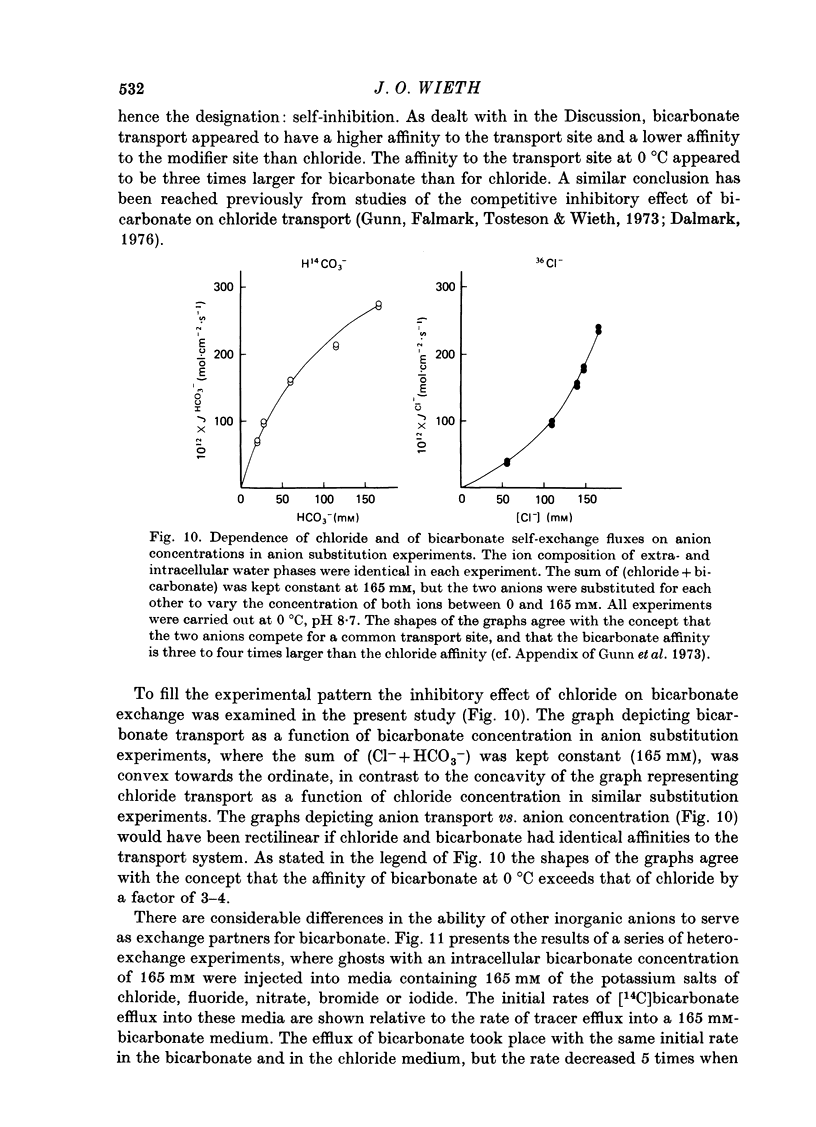






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brahm J. Temperature-dependent changes of chloride transport kinetics in human red cells. J Gen Physiol. 1977 Sep;70(3):283–306. doi: 10.1085/jgp.70.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Cass A., Dalmark M. Equilibrium dialysis of ions in nystatin-treated red cells. Nat New Biol. 1973 Jul 11;244(132):47–49. doi: 10.1038/newbio244047a0. [DOI] [PubMed] [Google Scholar]
- Chow E. I., Crandall E. D., Forster R. E. Kinetics of bicarbonate-chloride exchange across the human red blood cell membrane. J Gen Physiol. 1976 Dec;68(6):633–652. doi: 10.1085/jgp.68.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin J. L., Motais R. The role of carbonic anhydrase inhibitors on anion permeability into ox red blood cells. J Physiol. 1976 Mar;256(1):61–80. doi: 10.1113/jphysiol.1976.sp011311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall E. D., Obaid A. L., Forster R. E. Bicarbonate-chloride exchange in erythrocyte suspensions. Stopped-flow pH electrode measurements. Biophys J. 1978 Oct;24(1):35–47. doi: 10.1016/S0006-3495(78)85329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmark M. Effects of halides and bicarbonate on chloride transport in human red blood cells. J Gen Physiol. 1976 Feb;67(2):223–234. doi: 10.1085/jgp.67.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmark M., Wieth J. O. Temperature dependence of chloride, bromide, iodide, thiocyanate and salicylate transport in human red cells. J Physiol. 1972 Aug;224(3):583–610. doi: 10.1113/jphysiol.1972.sp009914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuticke B. Properties and structural basis of simple diffusion pathways in the erythrocyte membrane. Rev Physiol Biochem Pharmacol. 1977;78:1–97. doi: 10.1007/BFb0027721. [DOI] [PubMed] [Google Scholar]
- Funder J., Tosteson D. C., Wieth J. O. Effects of bicarbonate on lithium transport in human red cells. J Gen Physiol. 1978 Jun;71(6):721–746. doi: 10.1085/jgp.71.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder J., Wieth J. O. Chloride transport in human erythrocytes and ghosts: a quantitative comparison. J Physiol. 1976 Nov;262(3):679–698. doi: 10.1113/jphysiol.1976.sp011615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn R. B., Dalmark M., Tosteson D. C., Wieth J. O. Characteristics of chloride transport in human red blood cells. J Gen Physiol. 1973 Feb;61(2):185–206. doi: 10.1085/jgp.61.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht J., Bisson M. A., Tosteson F. C. Diffusion of carbon dioxide through lipid bilayer membranes: effects of carbonic anhydrase, bicarbonate, and unstirred layers. J Gen Physiol. 1977 Jun;69(6):779–794. doi: 10.1085/jgp.69.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder L. B., Hayes S. L. Diffusion of sulfonamides in aqueous buffers and into red cells. Mol Pharmacol. 1965 Nov;1(3):266–279. [PubMed] [Google Scholar]
- Hunter M. J. A quantitative estimate of the non-exchange-restricted chloride permeability of the human red cell. J Physiol. 1971 Oct;218 (Suppl):49P–50P. [PubMed] [Google Scholar]
- Hunter M. J. Human erythrocyte anion permeabilities measured under conditions of net charge transfer. J Physiol. 1977 Jun;268(1):35–49. doi: 10.1113/jphysiol.1977.sp011845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M. H., Stewart D. R. THE ROLE OF CARBONIC ANHYDRASE IN CERTAIN IONIC EXCHANGES INVOLVING THE ERYTHROCYTE. J Gen Physiol. 1942 Mar 20;25(4):539–552. doi: 10.1085/jgp.25.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert A., Lowe A. G. Chloride/bicarbonate exchange in human erythrocytes. J Physiol. 1978 Feb;275:51–63. doi: 10.1113/jphysiol.1978.sp012177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepke S., Fasold H., Pring M., Passow H. A study of the relationship between inhibition of anion exchange and binding to the red blood cell membrane of 4,4'-diisothiocyano stilbene-2,2'-disulfonic acid (DIDS) and its dihydro derivative (H2DIDS). J Membr Biol. 1976 Oct 20;29(1-2):147–177. doi: 10.1007/BF01868957. [DOI] [PubMed] [Google Scholar]
- Ship S., Shami Y., Breuer W., Rothstein A. Synthesis of tritiated 4,4'-diisothiocyano-2,2'-stilbene disulfonic acid ([3H]DIDS) and its covalent reaction with sites related to anion transport in human red blood cells. J Membr Biol. 1977 May 12;33(3-4):311–323. doi: 10.1007/BF01869522. [DOI] [PubMed] [Google Scholar]


