Abstract
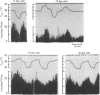
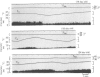
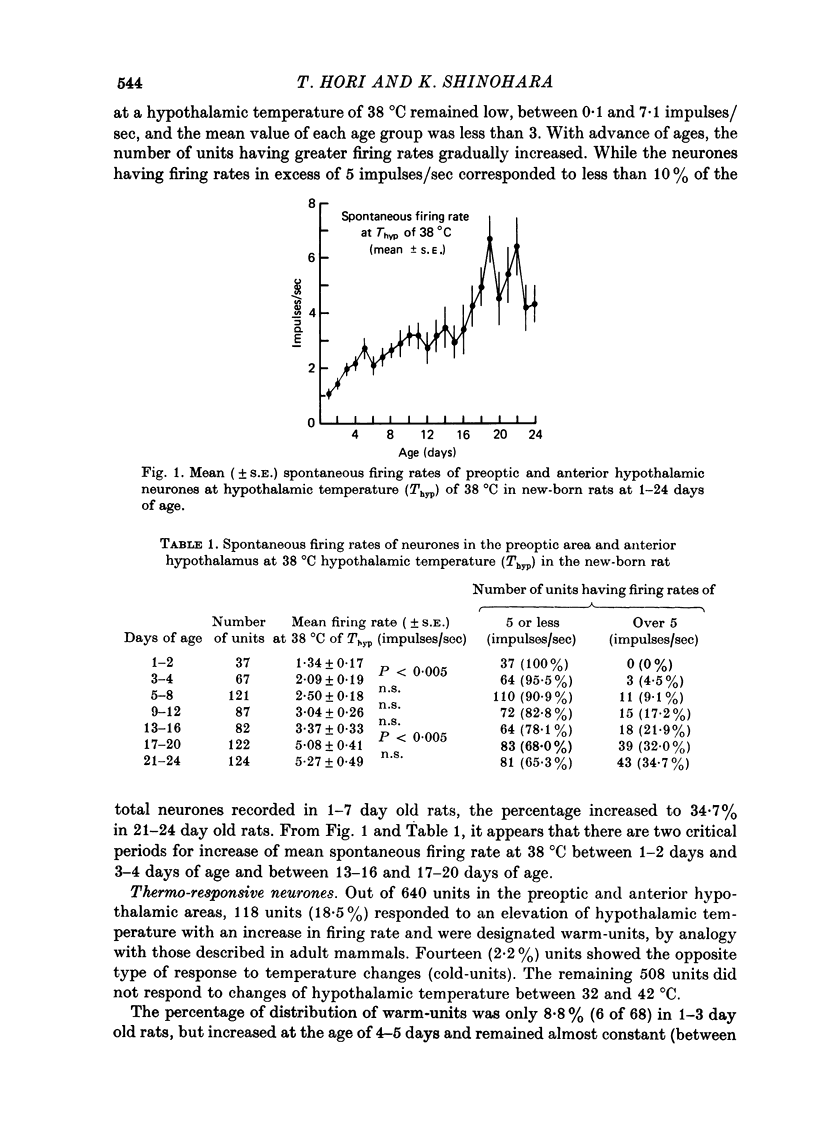
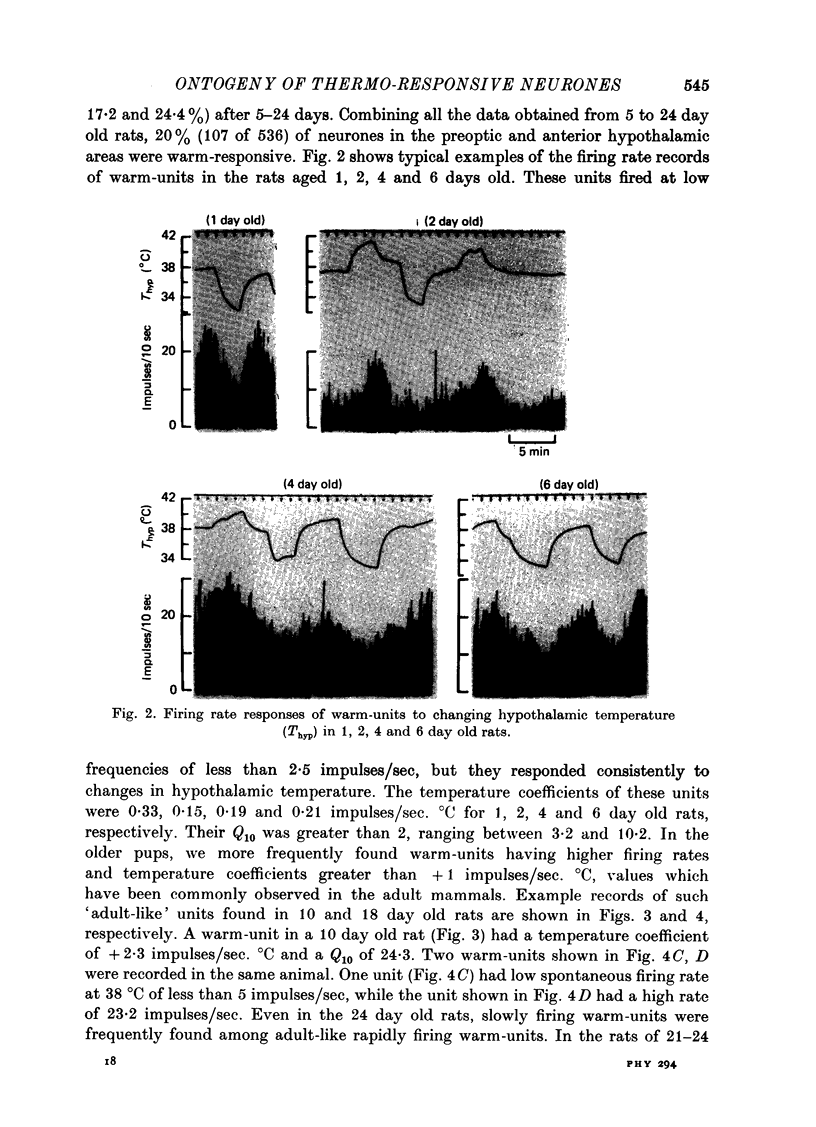
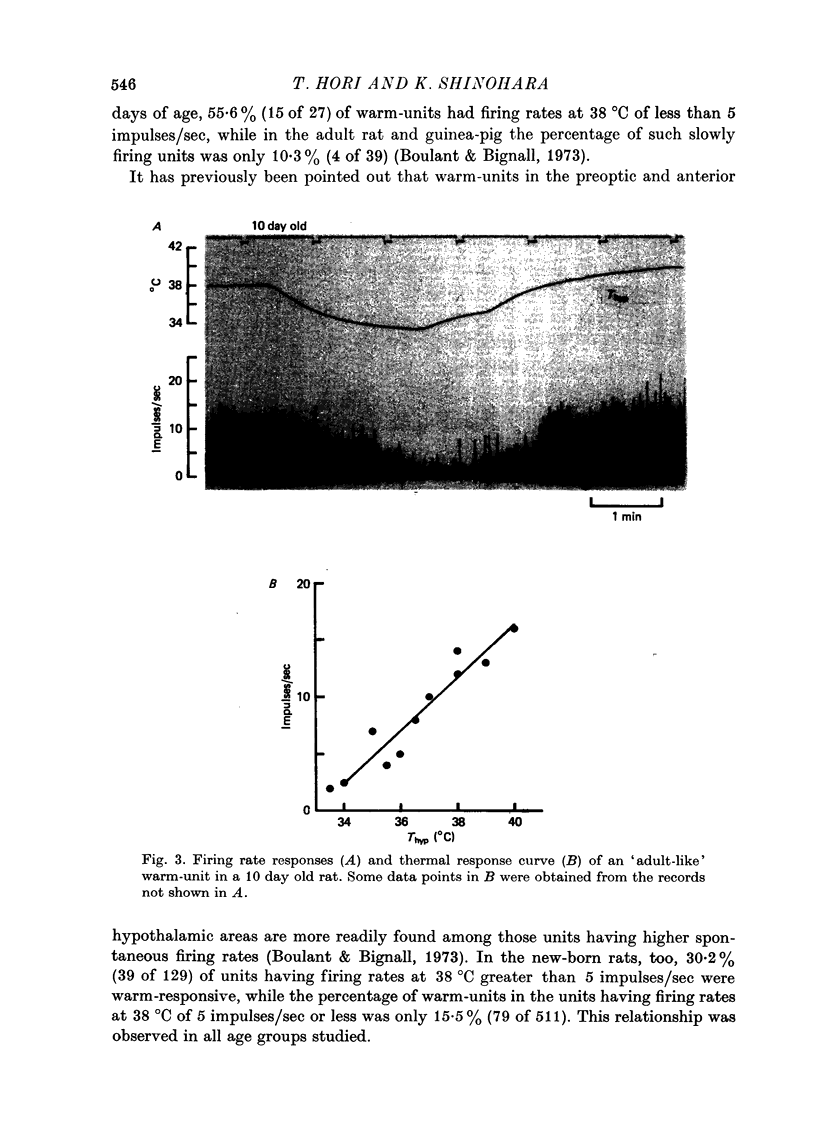
1. Single unit activities were recorded from the neurones in the preoptic area and anterior hypothalamus of developing new-born rats (aged 1-24 days old) during thermal stimulation of the brain. During the first 2 weeks of life, about 80% of these neurones had low spontaneous firing rates between 0.1 and 5 impulses/sec at 38 degrees C hypothalamic temperature (Thyp). 2. Out of 640 units studied, 118 units increased the firing rate upon elevation of Thyp (warm-units) and fourteen showed the opposite type of response to temperature changes (cold-units). Warm-units were found in the rats of all the age span studied and cold-units were recorded in the rats more than 8 days old. 3. Thermal coefficients of warm-units and cold-units varied between +0.11 and +2.47 and between -0.10 and -0.49 impulses/sec, degrees C, respectively. Number of warm-units with higher rates of firing and greater thermal coefficients, comparable to those of warm-units in the adult, gradually increased with growth. The thermal responsiveness of warm-units, when expressed by Q10, are already high even in the immediate neonatal period. Their Q10 values were in the range between 2 and 38.5 (mean 6.4). 4. Units responding to extrahypothalamic temperatures were only found in the rats more than 14 days old. 5. All the six warm-units tested increased the firing rates following subcutaneous injections of capsaicin, while the majority of thermo-unresponsive units were not affected by this drug. 6. It is suggested that thermo-responsive neurones in the preoptic area and anterior hypothalamus in the new-born rat have attained some degree of electrophysiological maturity, despite their slowly firing characteristics.
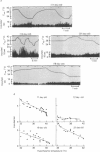
Full text
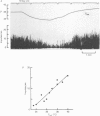
PDF


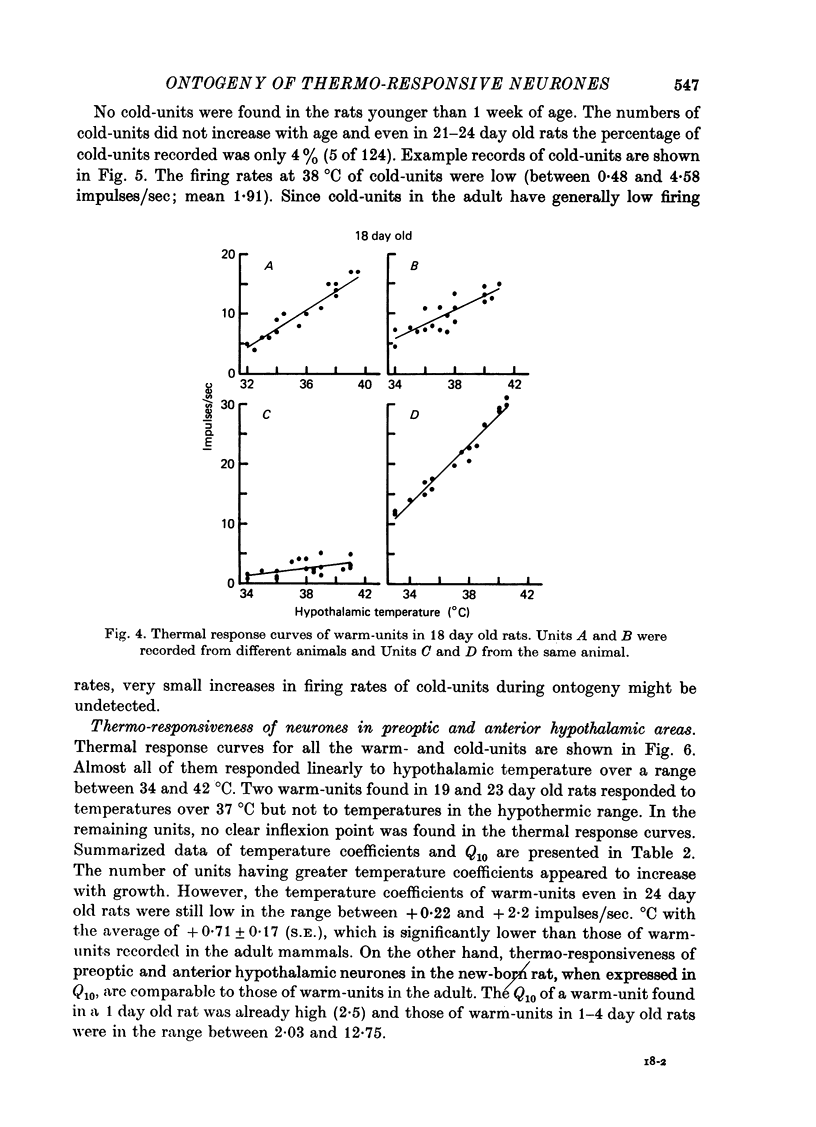
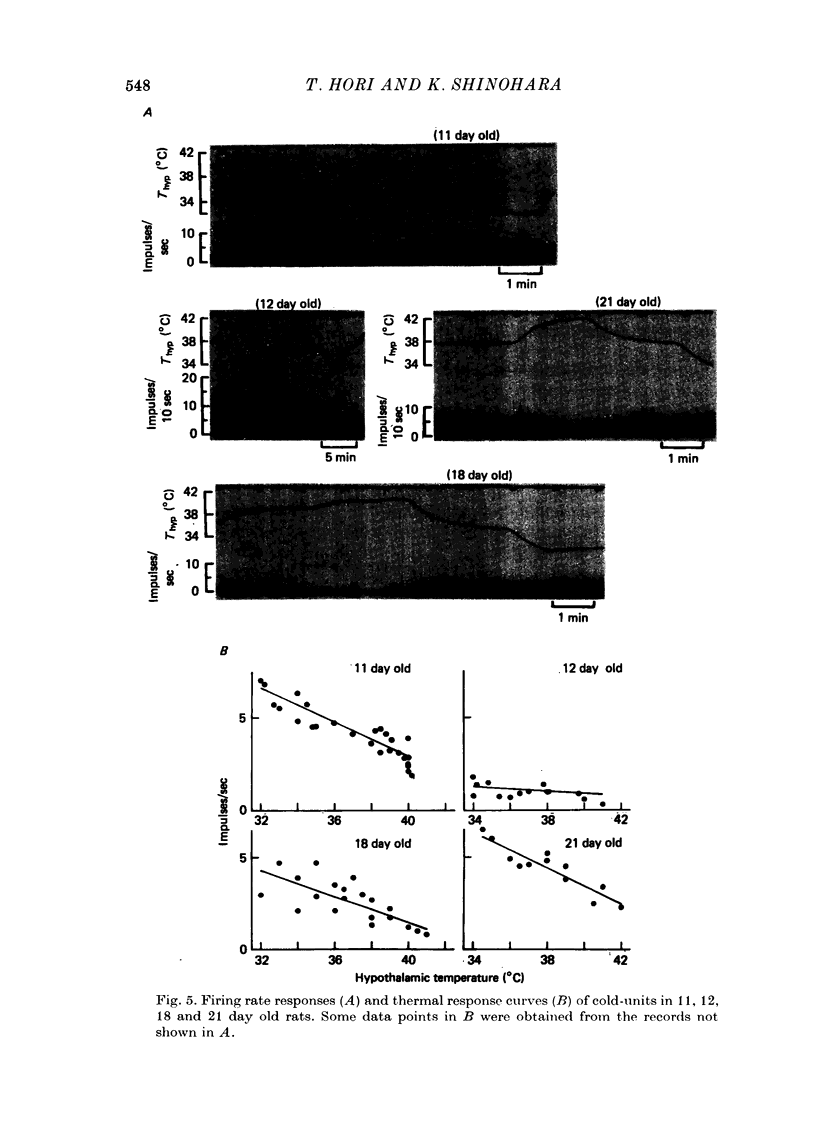
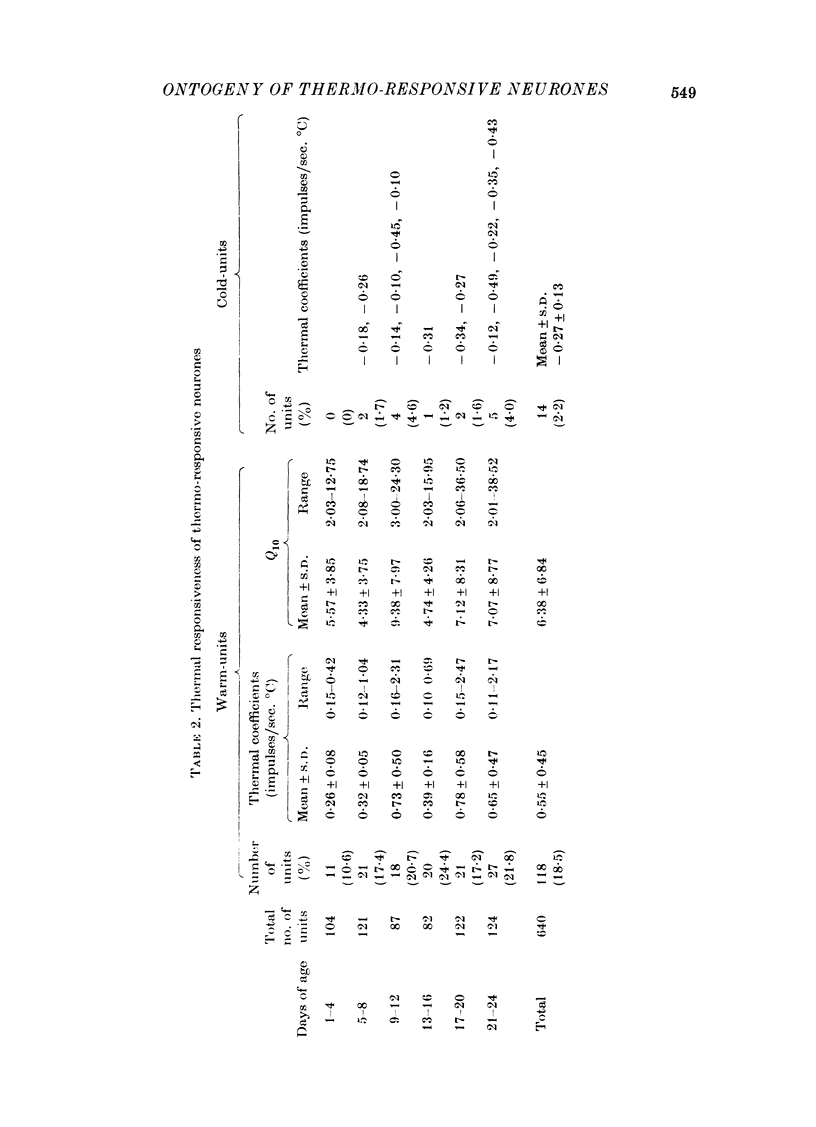
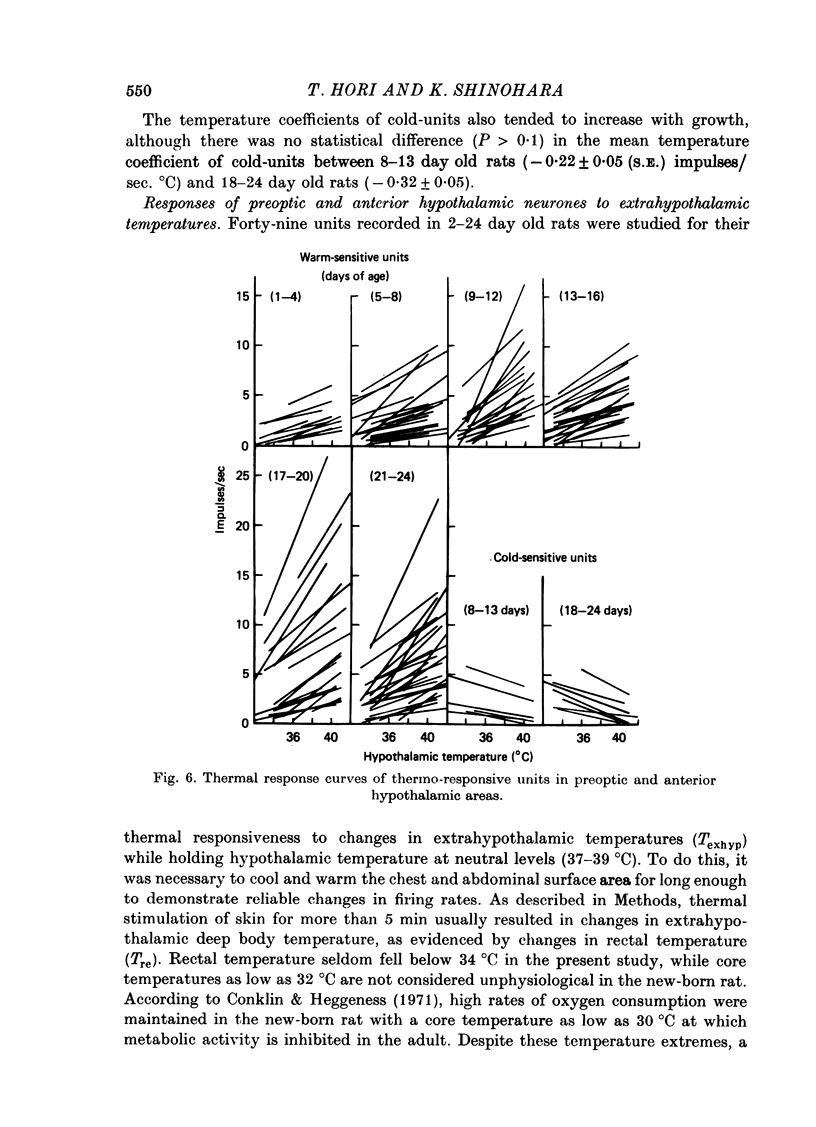
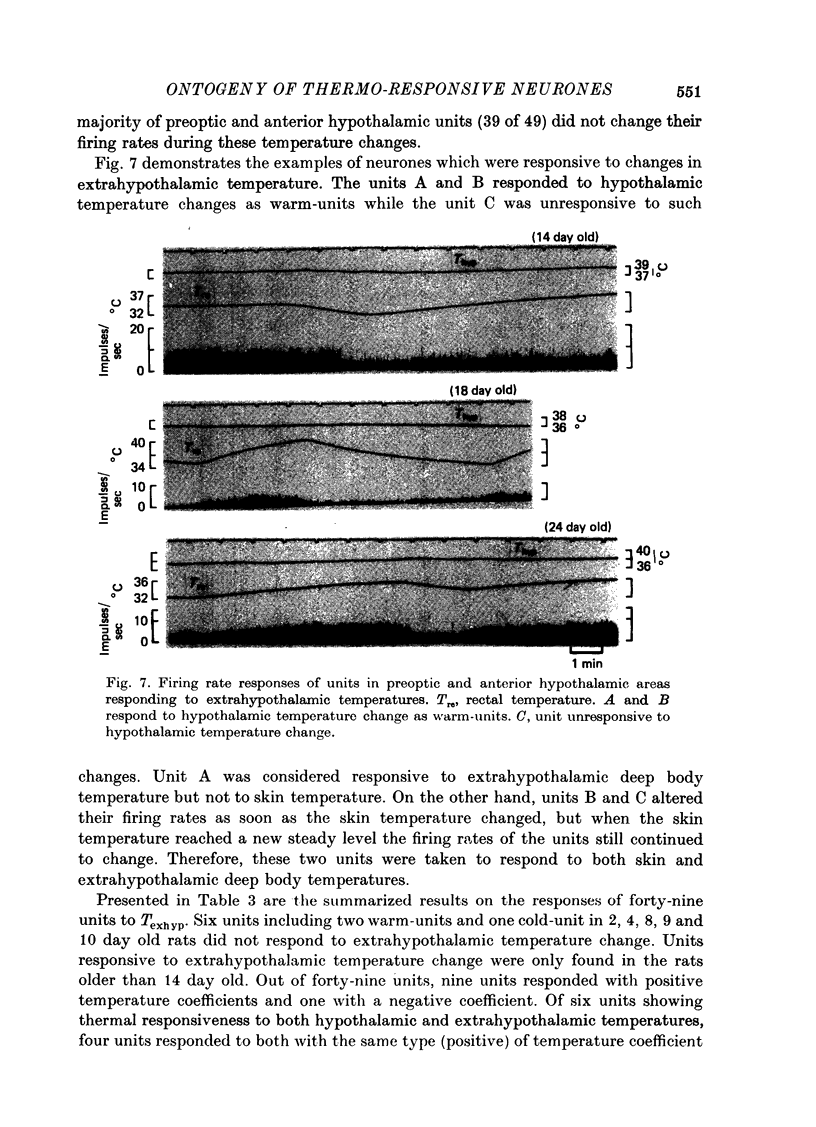
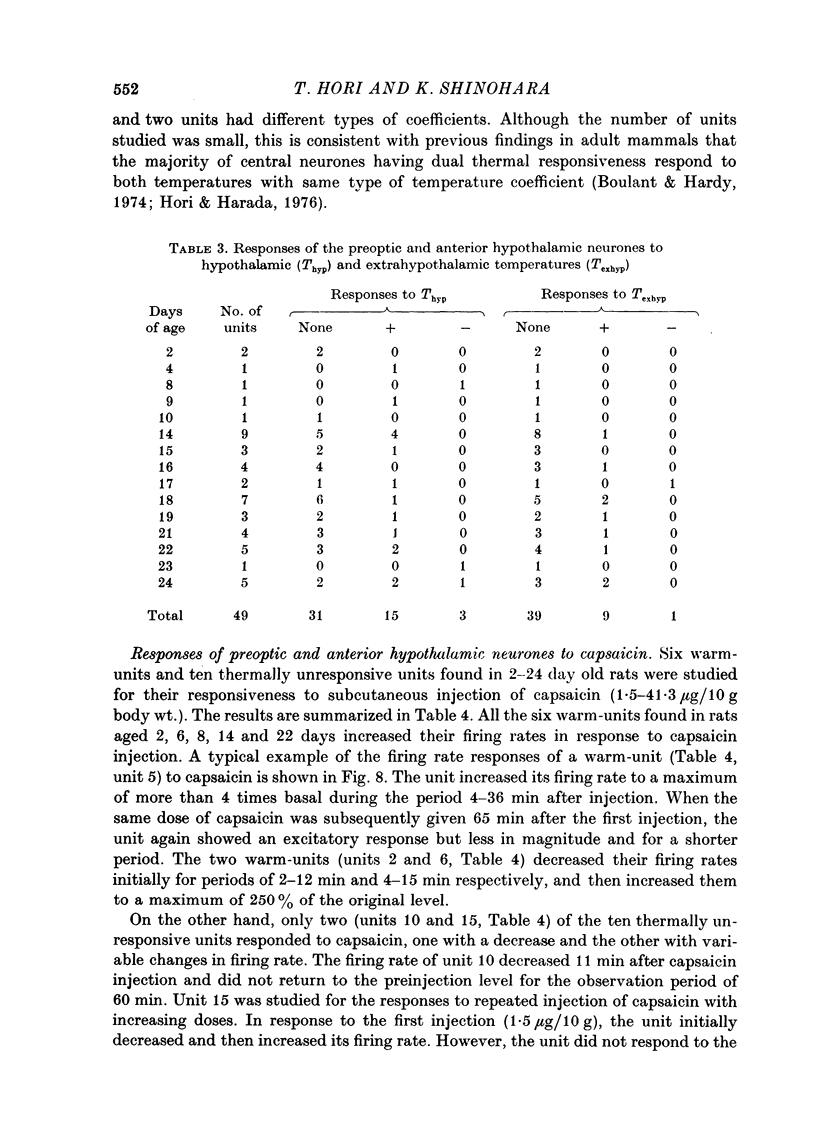
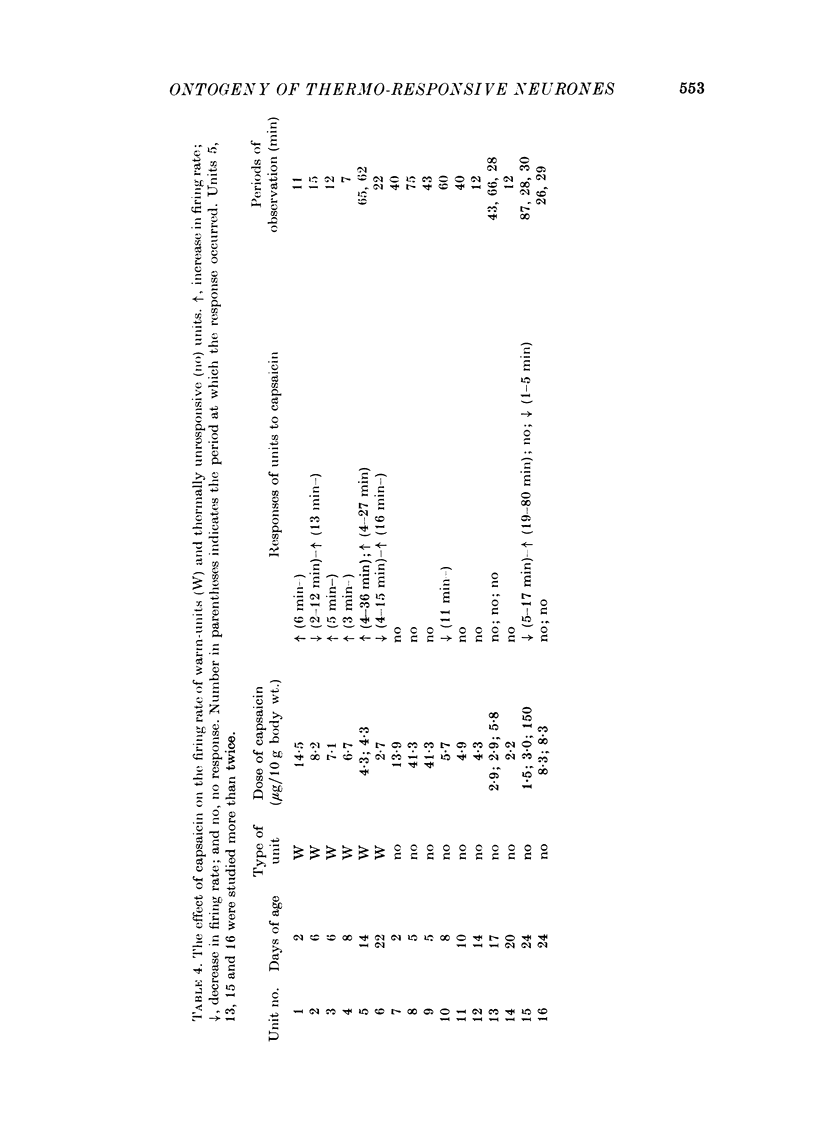
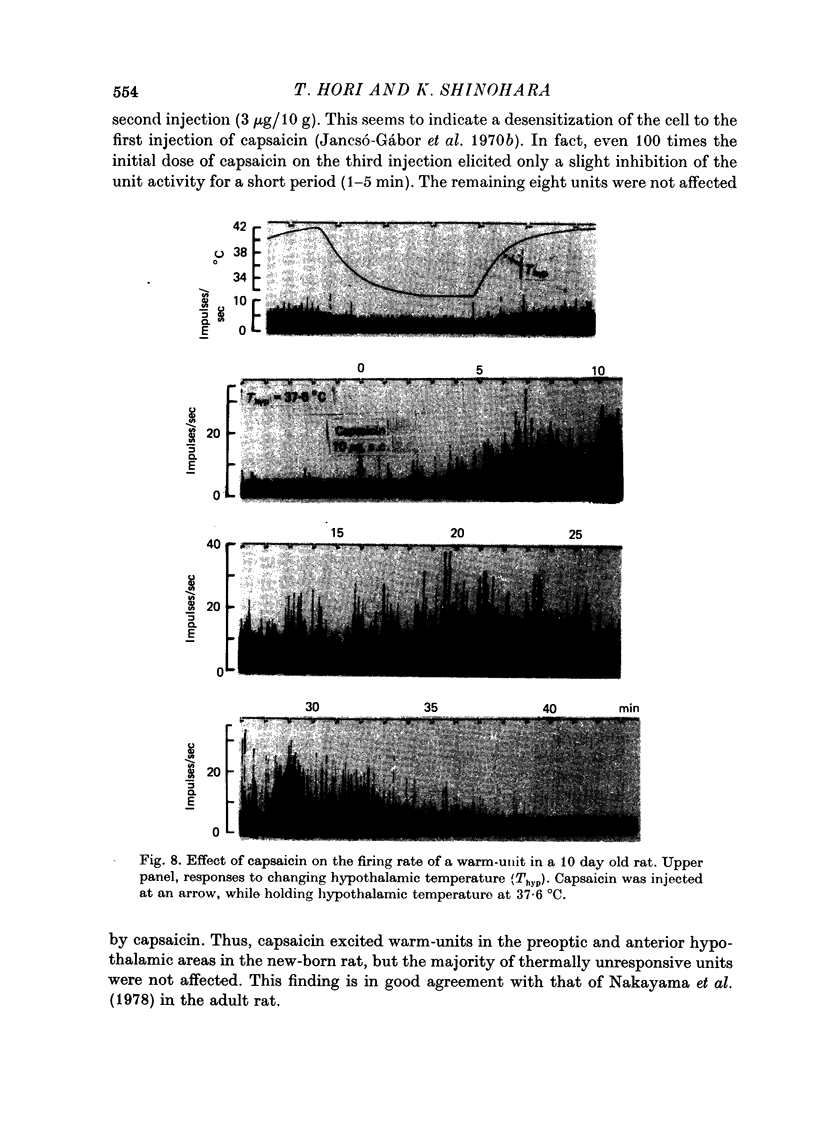






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almli C. R., McMullen N. T., Golden G. T. Infant rats: hypothalamic unit activity. Brain Res Bull. 1976 Nov-Dec;1(6):543–552. doi: 10.1016/0361-9230(76)90081-2. [DOI] [PubMed] [Google Scholar]
- Boulant J. A., Bignall K. E. Determinants of hypothalamic neuronal thermosensitivity in ground squirrels and rats. Am J Physiol. 1973 Aug;225(2):306–310. doi: 10.1152/ajplegacy.1973.225.2.306. [DOI] [PubMed] [Google Scholar]
- Boulant J. A., Hardy J. D. The effect of spinal and skin temperatures on the firing rate and thermosensitivity of preoptic neurones. J Physiol. 1974 Aug;240(3):639–660. doi: 10.1113/jphysiol.1974.sp010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COGGESHALL R. E. A STUDY OF DIENCEPHALIC DEVELOPMENT IN THE ALBINO RAT. J Comp Neurol. 1964 Apr;122:241–269. doi: 10.1002/cne.901220208. [DOI] [PubMed] [Google Scholar]
- Cabanac M., Hammel T., Hardy J. D. Tiliqua scincoides: temperature-sensitive units in lizard brain. Science. 1967 Nov;158(3804):1050–1051. doi: 10.1126/science.158.3804.1050. [DOI] [PubMed] [Google Scholar]
- Conklin P., Heggeness F. W. Maturation of tempeature homeostasis in the rat. Am J Physiol. 1971 Feb;220(2):333–336. doi: 10.1152/ajplegacy.1971.220.2.333. [DOI] [PubMed] [Google Scholar]
- Deza L., Eidelberg E. Development of cortical electrical activity in the rat. Exp Neurol. 1967 Apr;17(4):425–438. doi: 10.1016/0014-4886(67)90129-x. [DOI] [PubMed] [Google Scholar]
- Eisenman J. S., Jackson D. C. Thermal response patterns of septal and preoptic neurons in cats. Exp Neurol. 1967 Sep;19(1):33–45. doi: 10.1016/0014-4886(67)90005-2. [DOI] [PubMed] [Google Scholar]
- Fowler S. J., Kellogg C. Ontogeny of thermoregulatory mechanisms in the rat. J Comp Physiol Psychol. 1975 Sep;89(7):738–746. doi: 10.1037/h0077037. [DOI] [PubMed] [Google Scholar]
- Guieu J. D., Hardy J. D. Effects of heating and cooling of the spinal cord on preoptic unit activity. J Appl Physiol. 1970 Nov;29(5):675–683. doi: 10.1152/jappl.1970.29.5.675. [DOI] [PubMed] [Google Scholar]
- HARDY J. D., HELLON R. F., SUTHERLAND K. TEMPERATURE-SENSITIVE NEURONES IN THE DOG'S HYPOTHALAMUS. J Physiol. 1964 Dec;175:242–253. doi: 10.1113/jphysiol.1964.sp007515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F. The marking of electrode tip positions in nervous tissue. J Physiol. 1971;214 (Suppl):12P–12P. [PubMed] [Google Scholar]
- Henderston T. C., Luckwill R. G., Nayernouri T. Single unit responses to temperature change in the brain of newborn rabbits. Int J Biometeorol. 1971 Dec;15(2):309–312. doi: 10.1007/BF01803917. [DOI] [PubMed] [Google Scholar]
- Hori T., Harada Y. Midbrain neuronal responses to local and spinal cord temperatures. Am J Physiol. 1976 Nov;231(5 Pt 1):1573–1578. doi: 10.1152/ajplegacy.1976.231.5.1573. [DOI] [PubMed] [Google Scholar]
- Hori T., Nakayama T. Effects of biogenic amines on central thermoresponsive neurones in the rabbit. J Physiol. 1973 Jul;232(1):71–85. doi: 10.1113/jphysiol.1973.sp010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher P. R. Development of cortical neuronal activity in the neonatal cat. Exp Neurol. 1967 Mar;17(3):247–262. doi: 10.1016/0014-4886(67)90104-5. [DOI] [PubMed] [Google Scholar]
- Hyvärinen J. Analysis of spontaneous spike potential activity in developing rabbit diencephalon. Acta Physiol Scand Suppl. 1966;278:1–67. [PubMed] [Google Scholar]
- Ifft J. D. An autoradiographic study of the time of final division of neurons in rat hypothalamic nuclei. J Comp Neurol. 1972 Feb;144(2):193–204. doi: 10.1002/cne.901440204. [DOI] [PubMed] [Google Scholar]
- Jahns R., Werner J. Special aspects of firing rates and thermosensitivity of preoptic neurons. Exp Brain Res. 1974;21(1):107–112. doi: 10.1007/BF00234261. [DOI] [PubMed] [Google Scholar]
- Jancsó-Gábor A., Szolcsányi J., Jancsó N. Irreversible impairment of thermoregulation induced by capsaicin and similar pungent substances in rats and guinea-pigs. J Physiol. 1970 Mar;206(3):495–507. doi: 10.1113/jphysiol.1970.sp009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancsó-Gábor A., Szolcsányi J., Jancsó N. Stimulation and desensitization of the hypothalamic heat-sensitive structures by capsaicin in rats. J Physiol. 1970 Jun;208(2):449–459. doi: 10.1113/jphysiol.1970.sp009130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizou L. A. The postnatal ontogeny of monoamine-containing neurones in the central nervous system of the albino rat. Brain Res. 1972 May 26;40(2):395–418. doi: 10.1016/0006-8993(72)90142-4. [DOI] [PubMed] [Google Scholar]
- Reier P. J., Cullen M. J., Froelich J. S., Rothchild I. The ultrastructure of the developing medial preoptic nucleus in the postnatal rat. Brain Res. 1977 Feb 25;122(3):415–436. doi: 10.1016/0006-8993(77)90454-1. [DOI] [PubMed] [Google Scholar]
- TAYLOR P. M. Oxygen consumption in new-born rats. J Physiol. 1960 Nov;154:153–168. doi: 10.1113/jphysiol.1960.sp006570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G. E., Moore R. E. A study of newborn rats exposed to the cold. Can J Physiol Pharmacol. 1968 Nov;46(6):865–871. doi: 10.1139/y68-135. [DOI] [PubMed] [Google Scholar]
- Torda C. Bioelectric observations on adult and newborn hypothalamic dendrites. J Neurosci Res. 1976;2(1):21–29. doi: 10.1002/jnr.490020104. [DOI] [PubMed] [Google Scholar]