Abstract
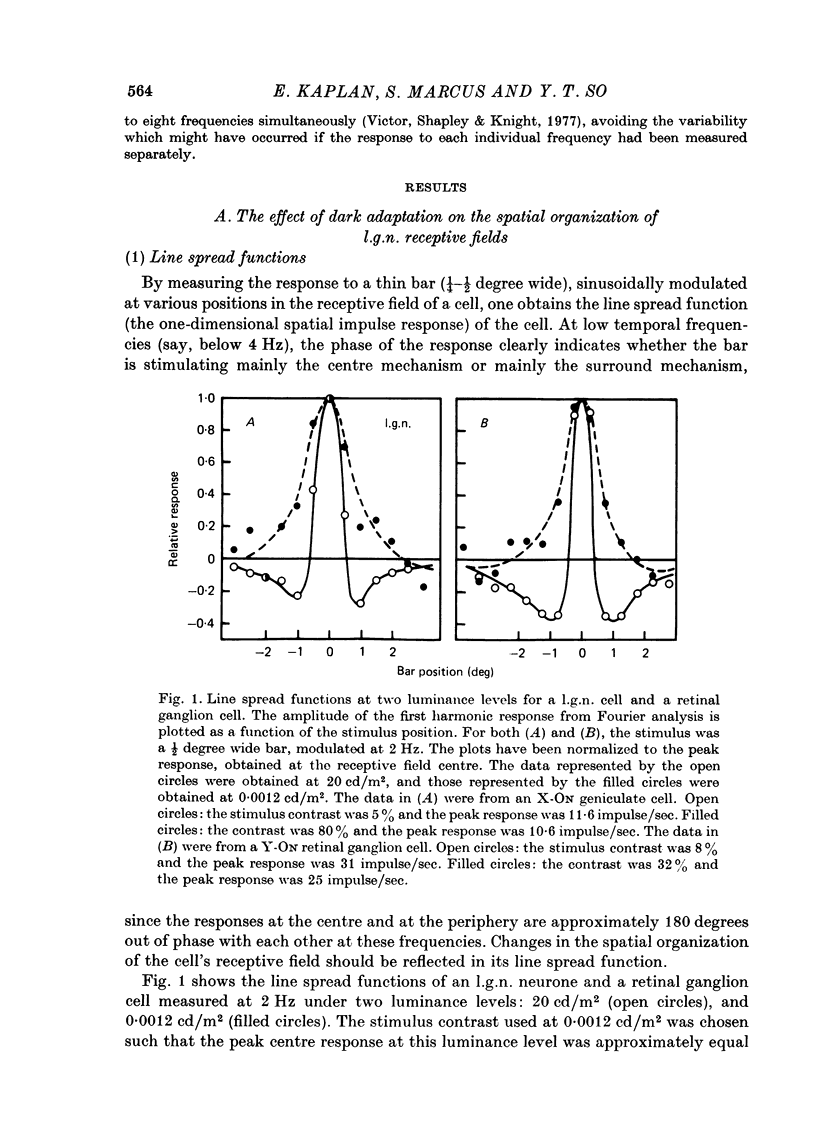
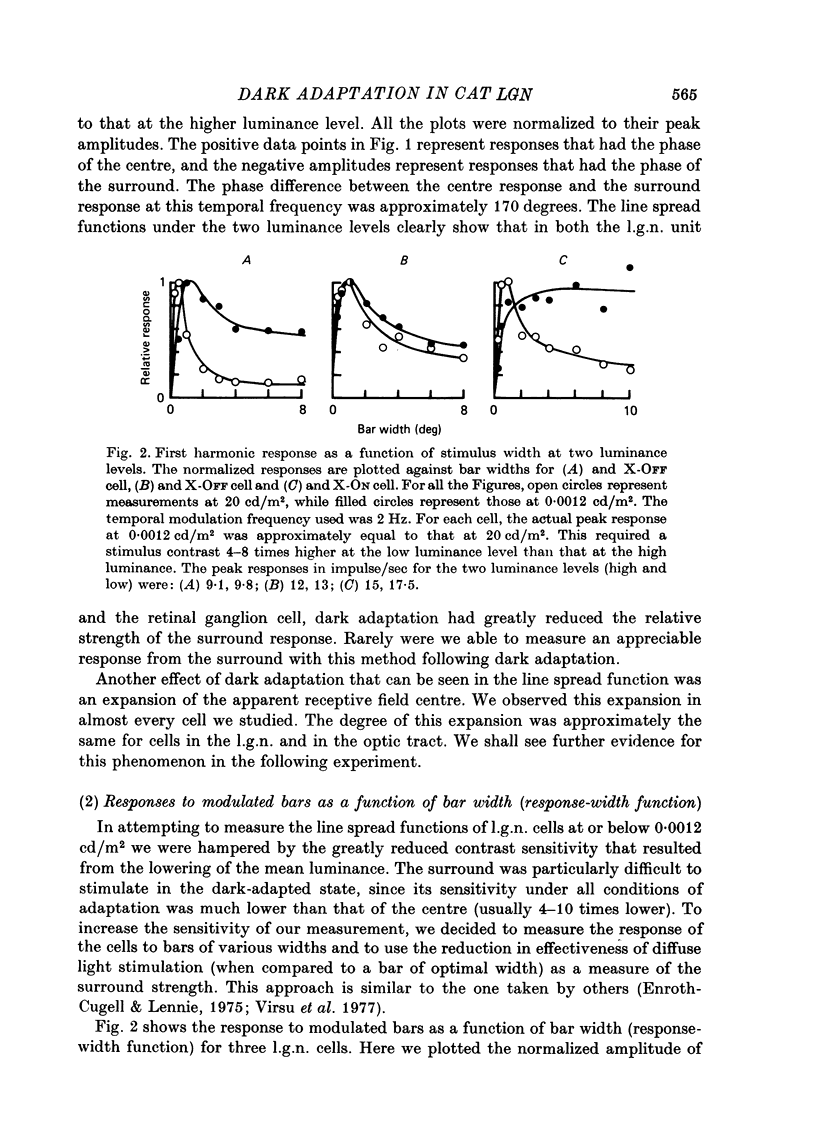
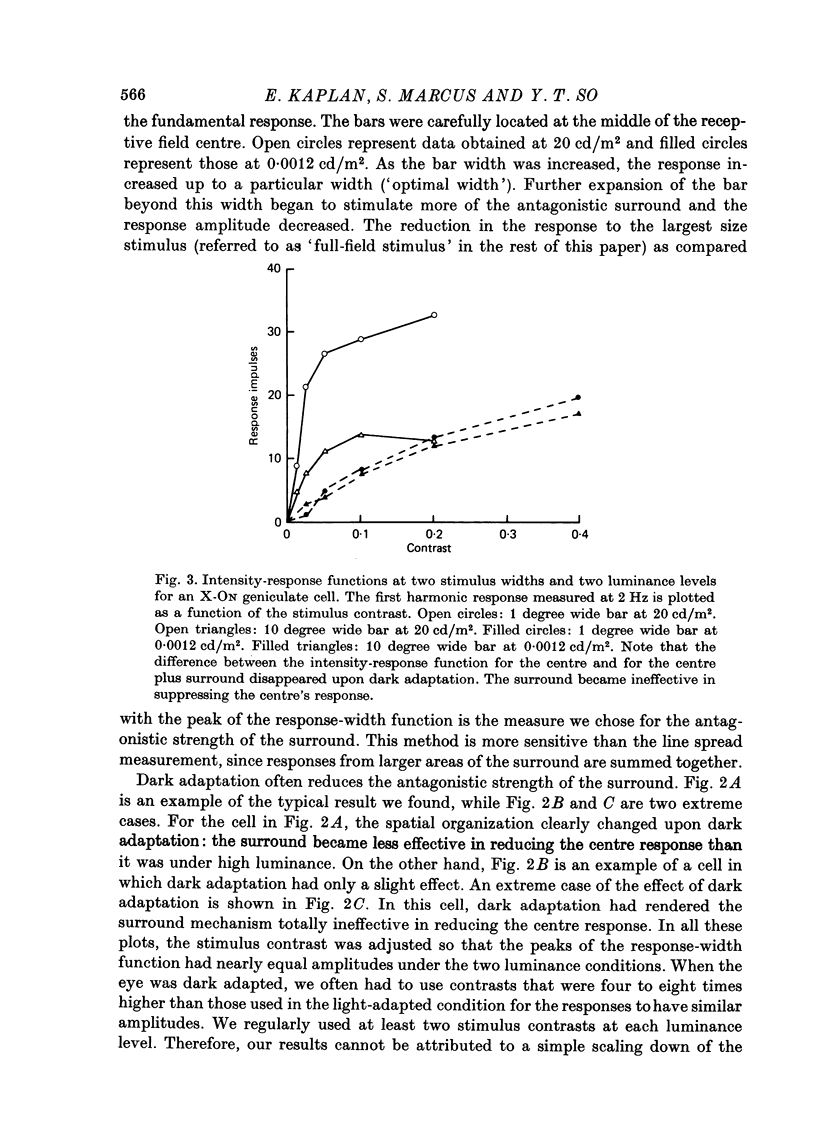
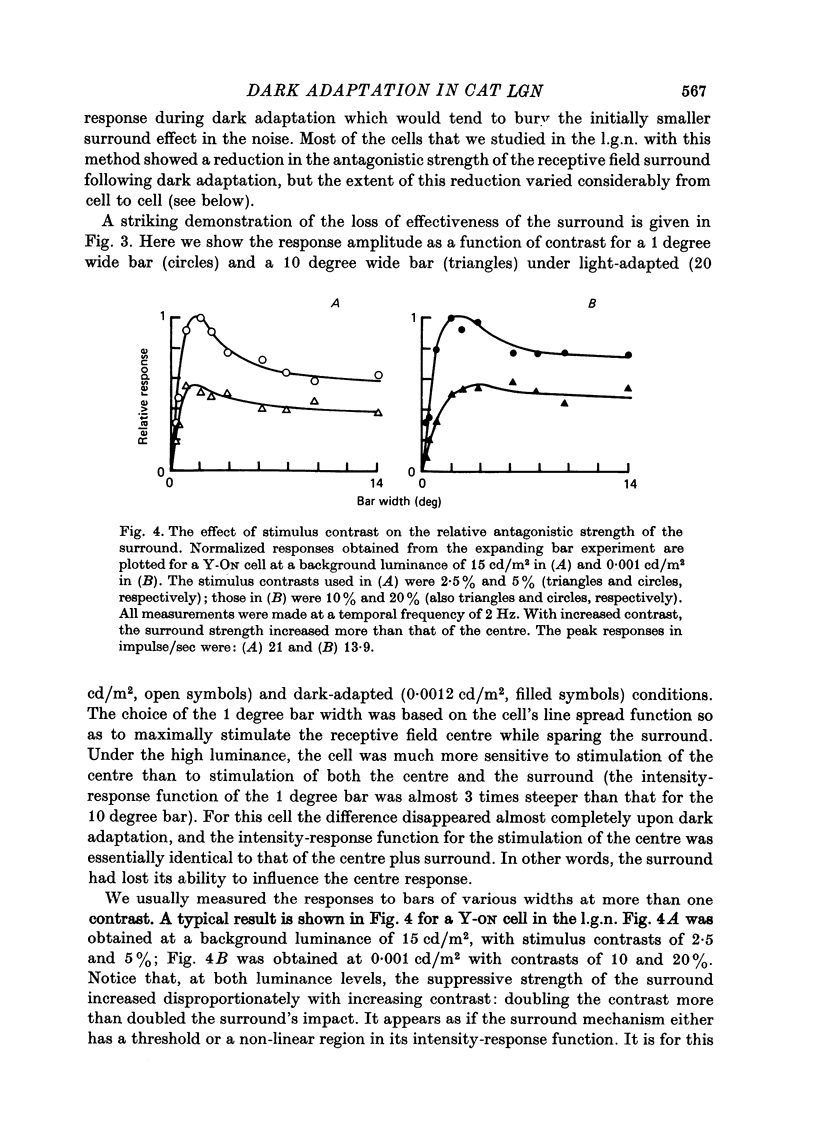
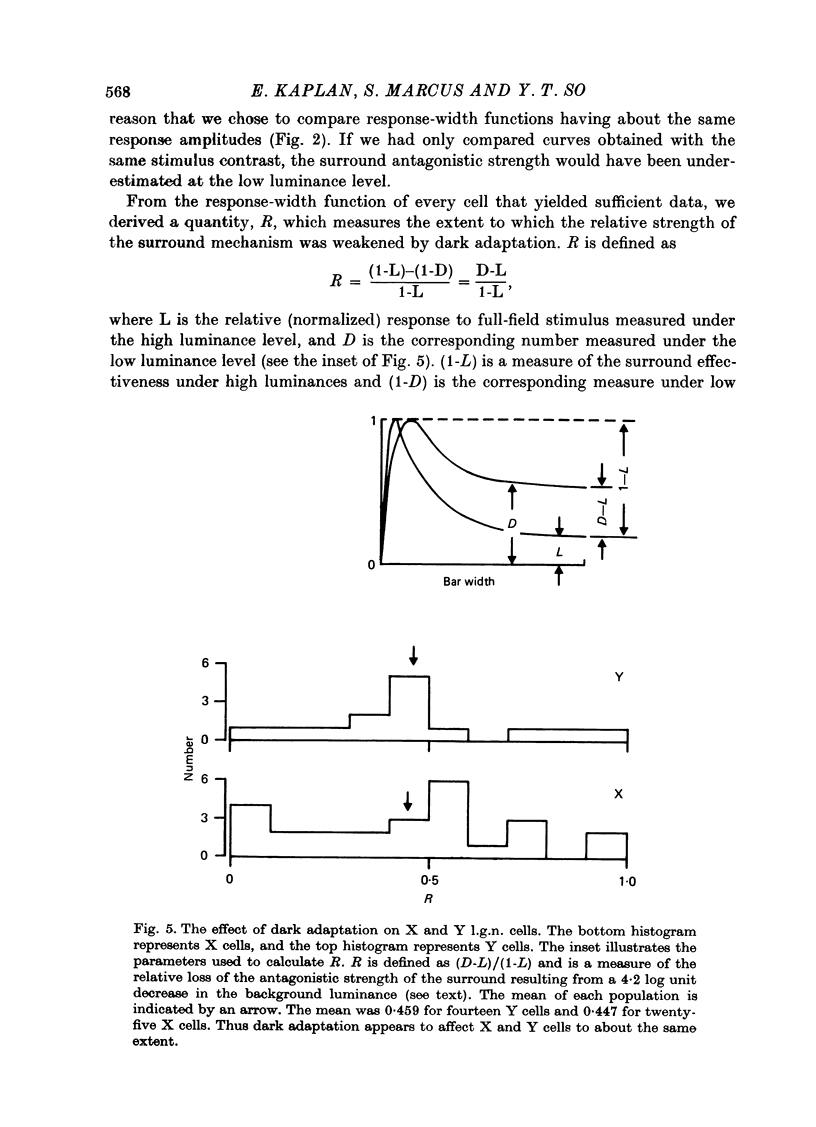
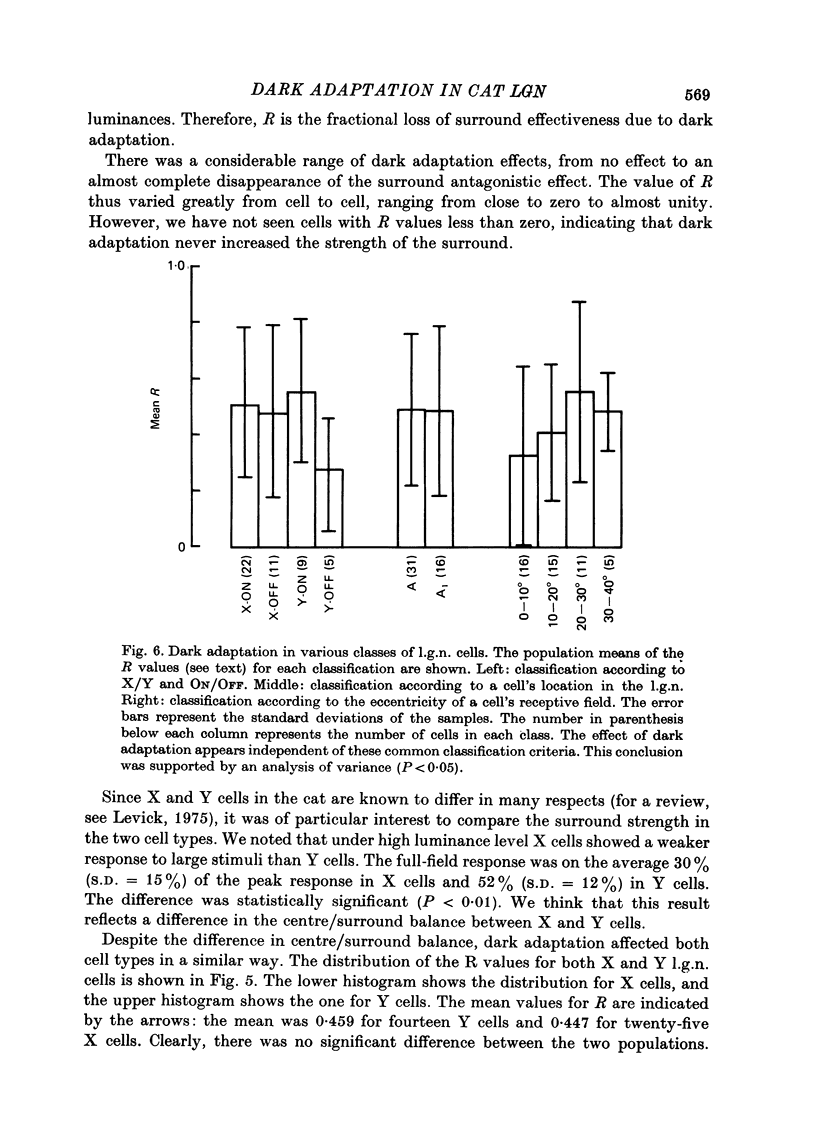
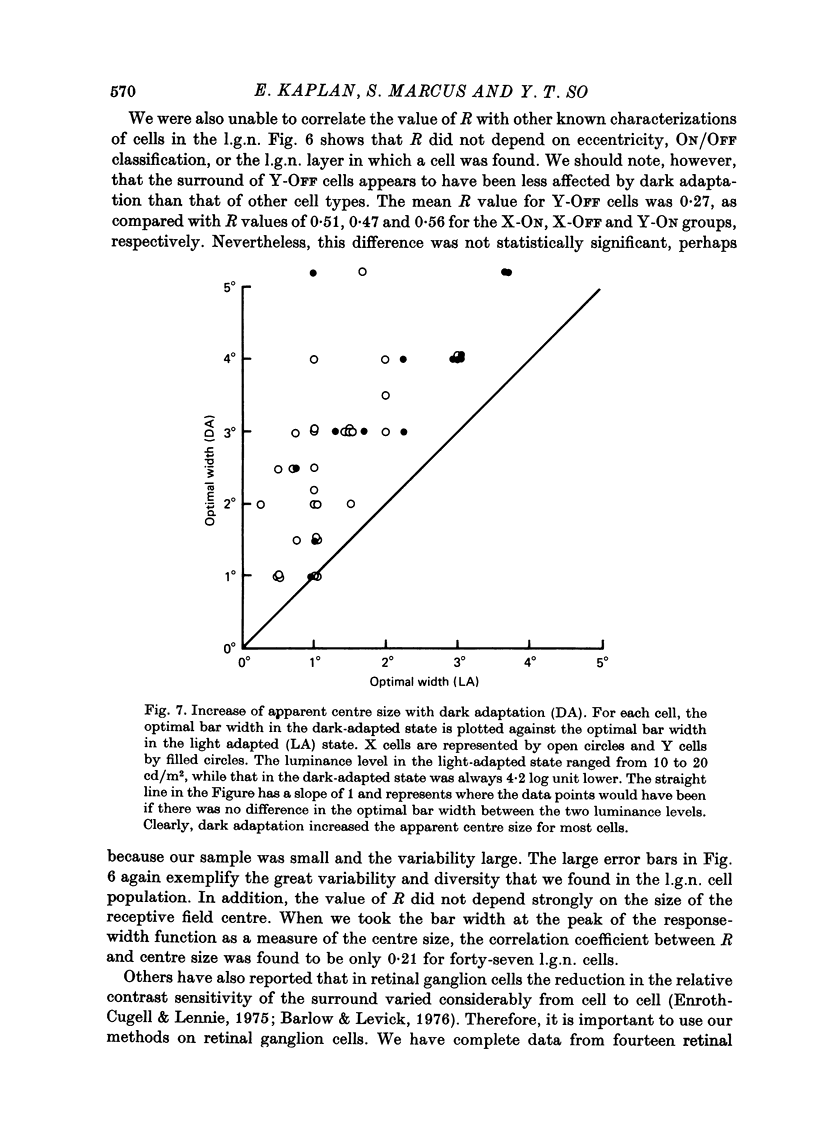
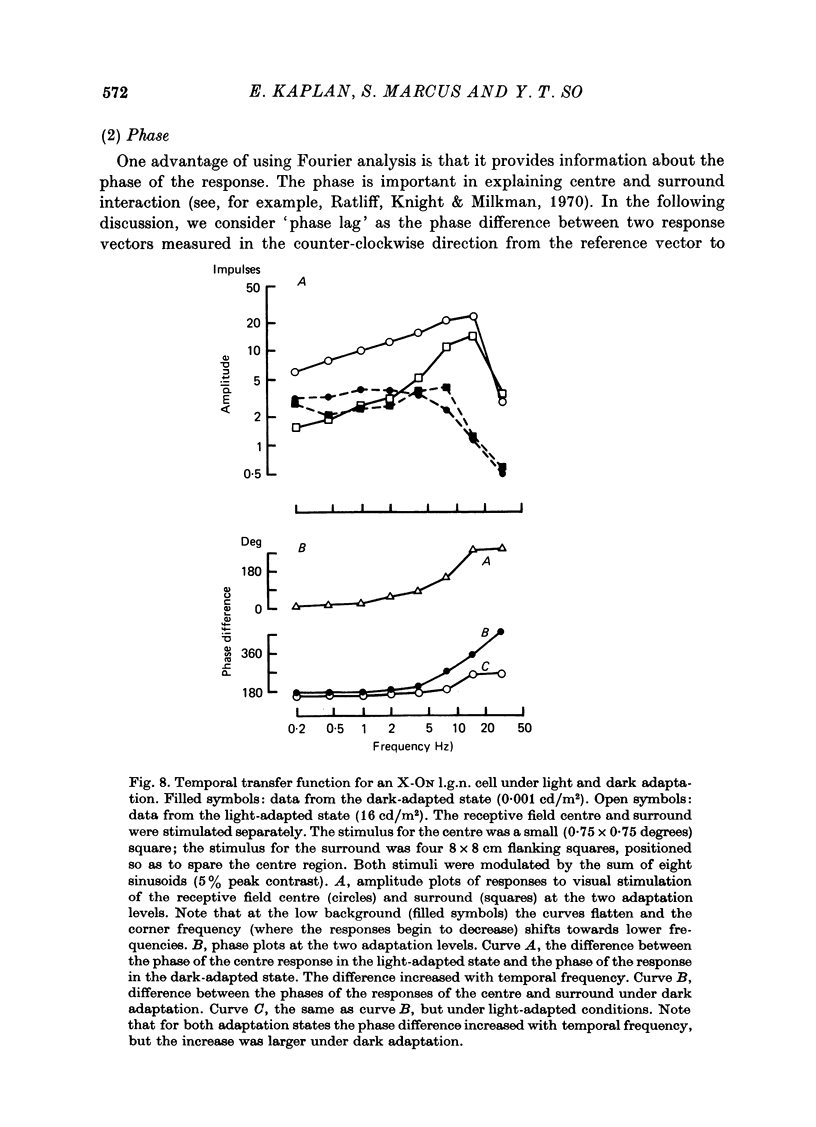
1. We studied the effect of dark adaptation on the spatial organization of receptive fields of single cells in the lateral geniculate nucleus l.g.n. of the cat. 2. Contrary to previous reports, we found that in many l.g.n. cells the ability of the receptive field surround to suppress the response of the centre was diminished following dark adaptation. 3. The degree of reduction of the surround antagonistic strength varied from cell to cell, and was independent of the various classifications of visual neurones (X/Y, ON/OFF, layer, A/layer, A1 and central/peripheral). 4. Most cells also showed an increase in the apparent size of the excitatory centre upon dark adaptation. On the average, the width of the most effective bar stimulus located at the centre of the receptive field increased more than twofold. 5. We also studied the effect of dark adaptation on the temporal properties of l.g.n. receptive fields. In many cells dark adaptation changed the temporal modulation transfer function: it flattened the amplitude function, and changed the phase relationship between the centre response and the surround response. 6. Retinal ganglion cells showed qualitatively similar behaviour to that of l.g.n. neurones. 7. Our data do not support the notion that retinal ganglion cell centres converge on l.g.n. cells to form their surround mechanism.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP P. O., BURKE W., DAVIS R. The identification of single units in central visual pathways. J Physiol. 1962 Aug;162:409–431. doi: 10.1113/jphysiol.1962.sp006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. The interpretation of the extracellular response of single lateral geniculate cells. J Physiol. 1962 Aug;162:451–472. doi: 10.1113/jphysiol.1962.sp006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
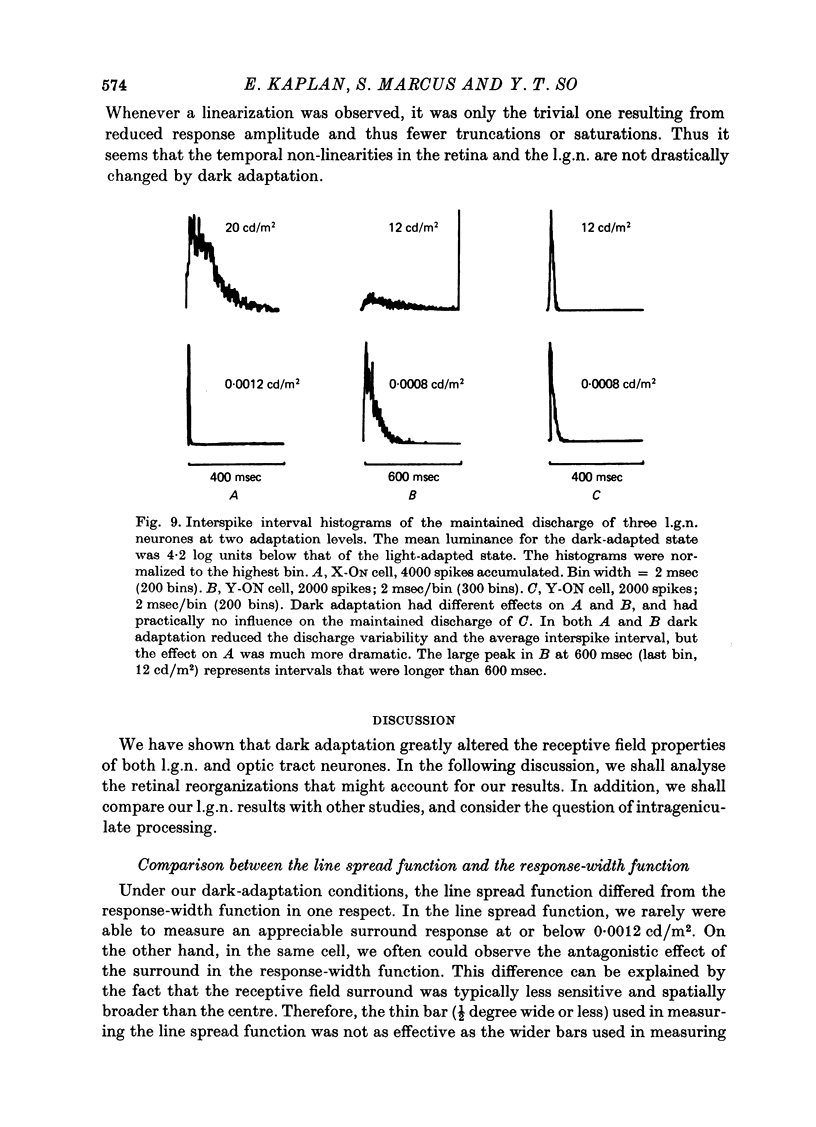
- Barlow H. B., Levick W. R. Threshold setting by the surround of cat retinal ganglion cells. J Physiol. 1976 Aug;259(3):737–757. doi: 10.1113/jphysiol.1976.sp011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
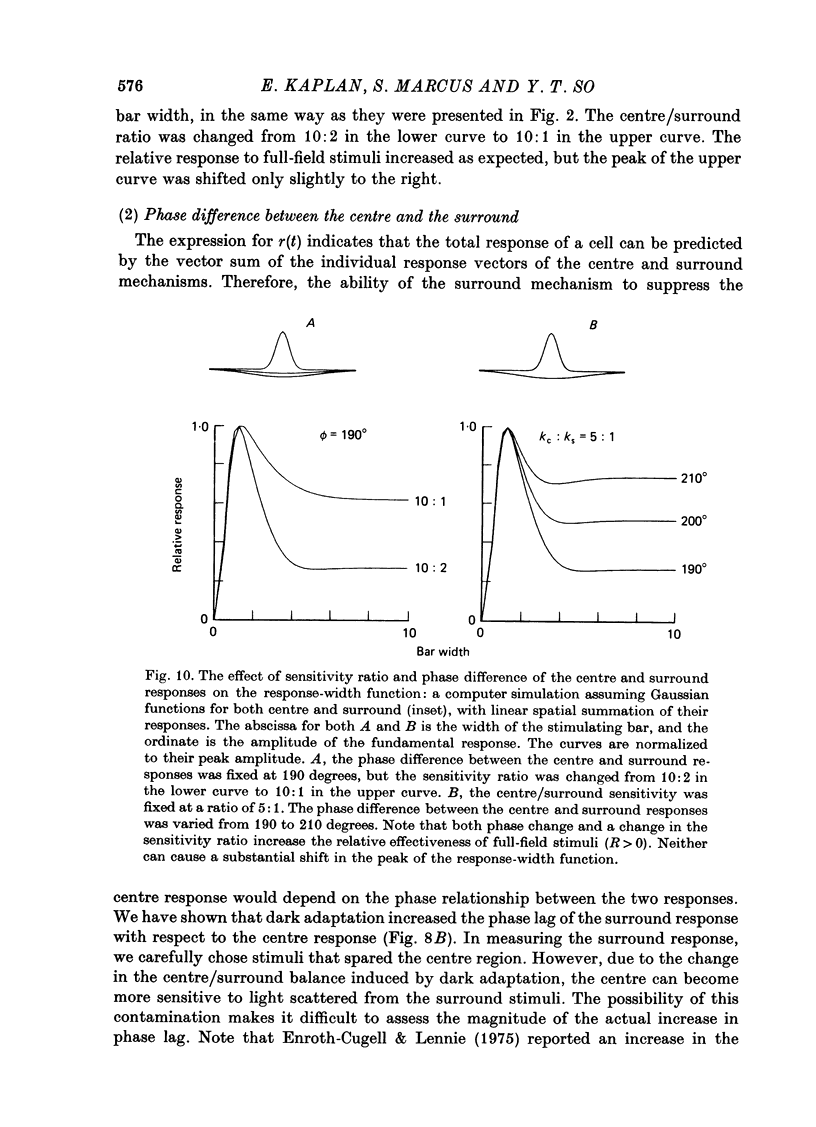
- Cleland B. G., Dubin M. W., Levick W. R. Simultaneous recording of input and output of lateral geniculate neurones. Nat New Biol. 1971 Jun 9;231(23):191–192. doi: 10.1038/newbio231191a0. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin M. W., Cleland B. G. Organization of visual inputs to interneurons of lateral geniculate nucleus of the cat. J Neurophysiol. 1977 Mar;40(2):410–427. doi: 10.1152/jn.1977.40.2.410. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Lennie P. The control of retinal ganglion cell discharge by receptive field surrounds. J Physiol. 1975 Jun;247(3):551–578. doi: 10.1113/jphysiol.1975.sp010947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. H. Properties of the surround response mechanism of cat retinal ganglion cells and centre-surround interaction. J Physiol. 1972 Jan;220(2):403–439. doi: 10.1113/jphysiol.1972.sp009714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Adaptation and dynamics of cat retinal ganglion cells. J Physiol. 1973 Sep;233(2):271–309. doi: 10.1113/jphysiol.1973.sp010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Flux, not retinal illumination, is what cat retinal ganglion cells really care about. J Physiol. 1973 Sep;233(2):311–326. doi: 10.1113/jphysiol.1973.sp010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald R., Chase R. An improved method for plotting retinal landmarks and focusing the eyes. Vision Res. 1971 Jan;11(1):95–96. doi: 10.1016/0042-6989(71)90207-0. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Integrative action in the cat's lateral geniculate body. J Physiol. 1961 Feb;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K. P., Stone J., Sherman S. M. Relay of receptive-field properties in dorsal lateral geniculate nucleus of the cat. J Neurophysiol. 1972 Jul;35(4):518–531. doi: 10.1152/jn.1972.35.4.518. [DOI] [PubMed] [Google Scholar]
- Kolb H., Famiglietti E. V. Rod and cone pathways in the inner plexiform layer of cat retina. Science. 1974 Oct 4;186(4158):47–49. doi: 10.1126/science.186.4158.47. [DOI] [PubMed] [Google Scholar]
- Levick W. R., Cleland B. G., Dubin M. W. Lateral geniculate neurons of cat: retinal inputs and physiology. Invest Ophthalmol. 1972 May;11(5):302–311. [PubMed] [Google Scholar]
- Levick W. R. Form and function of cat retinal ganglion cells. Nature. 1975 Apr 24;254(5502):659–662. doi: 10.1038/254659a0. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. Retinogeniculate convergence and analysis of contrast. J Neurophysiol. 1972 Jan;35(1):65–72. doi: 10.1152/jn.1972.35.1.65. [DOI] [PubMed] [Google Scholar]
- Poggio G. F., Baker F. H., Lamarre Y., Sanseverino E. R. Afferent inhibition at input to visual cortex of the cat. J Neurophysiol. 1969 Nov;32(6):892–915. doi: 10.1152/jn.1969.32.6.892. [DOI] [PubMed] [Google Scholar]
- Ratliff F., Knight B. W., Milkman N. Superposition of excitatory and inhibitory influences in the retina of Limulus: effect of delayed inhibition. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1558–1564. doi: 10.1073/pnas.67.3.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W. Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vision Res. 1965 Dec;5(11):583–601. doi: 10.1016/0042-6989(65)90033-7. [DOI] [PubMed] [Google Scholar]
- Shapley R. M., Victor J. D. The effect of contrast on the transfer properties of cat retinal ganglion cells. J Physiol. 1978 Dec;285:275–298. doi: 10.1113/jphysiol.1978.sp012571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R., Hochstein S. Visual spatial summation in two classes of geniculate cells. Nature. 1975 Jul 31;256(5516):411–413. doi: 10.1038/256411a0. [DOI] [PubMed] [Google Scholar]
- Singer W., Creutzfeldt O. D. Reciprocal lateral inhibition of on- and off-center neurones in the lateral geniculate body of the cat. Exp Brain Res. 1970;10(3):311–330. doi: 10.1007/BF00235054. [DOI] [PubMed] [Google Scholar]
- Singer W., Pöppel E., Creutzfeldt O. Inhibitory interaction in the cat's lateral geniculate nucleus. Exp Brain Res. 1972;14(2):210–226. doi: 10.1007/BF00234800. [DOI] [PubMed] [Google Scholar]
- Victor J. D., Shapley R. M., Knight B. W. Nonlinear analysis of cat retinal ganglion cells in the frequency domain. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3068–3072. doi: 10.1073/pnas.74.7.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virsu V., Lee B. B., Creutzfeldt O. D. Dark adaptation and receptive field organisation of cells in the cat lateral geniculate nucleus. Exp Brain Res. 1977 Jan 18;27(1):35–50. doi: 10.1007/BF00234823. [DOI] [PubMed] [Google Scholar]


