Abstract
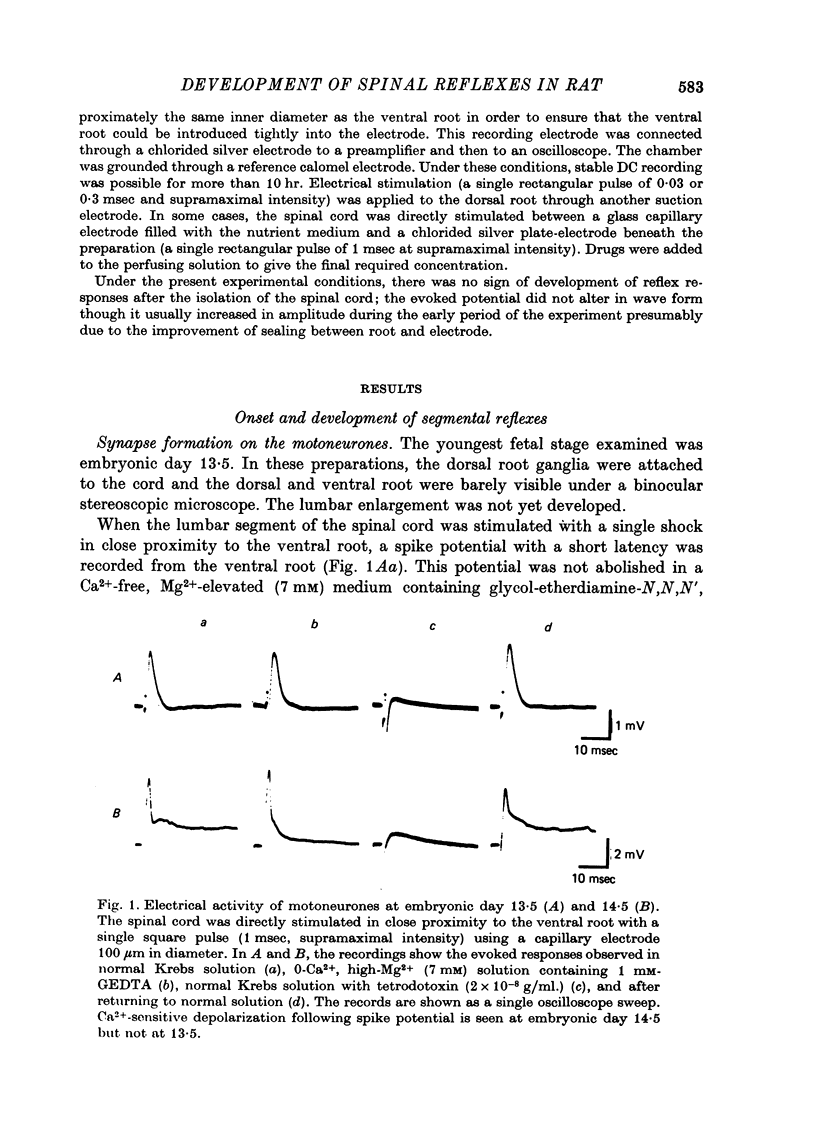
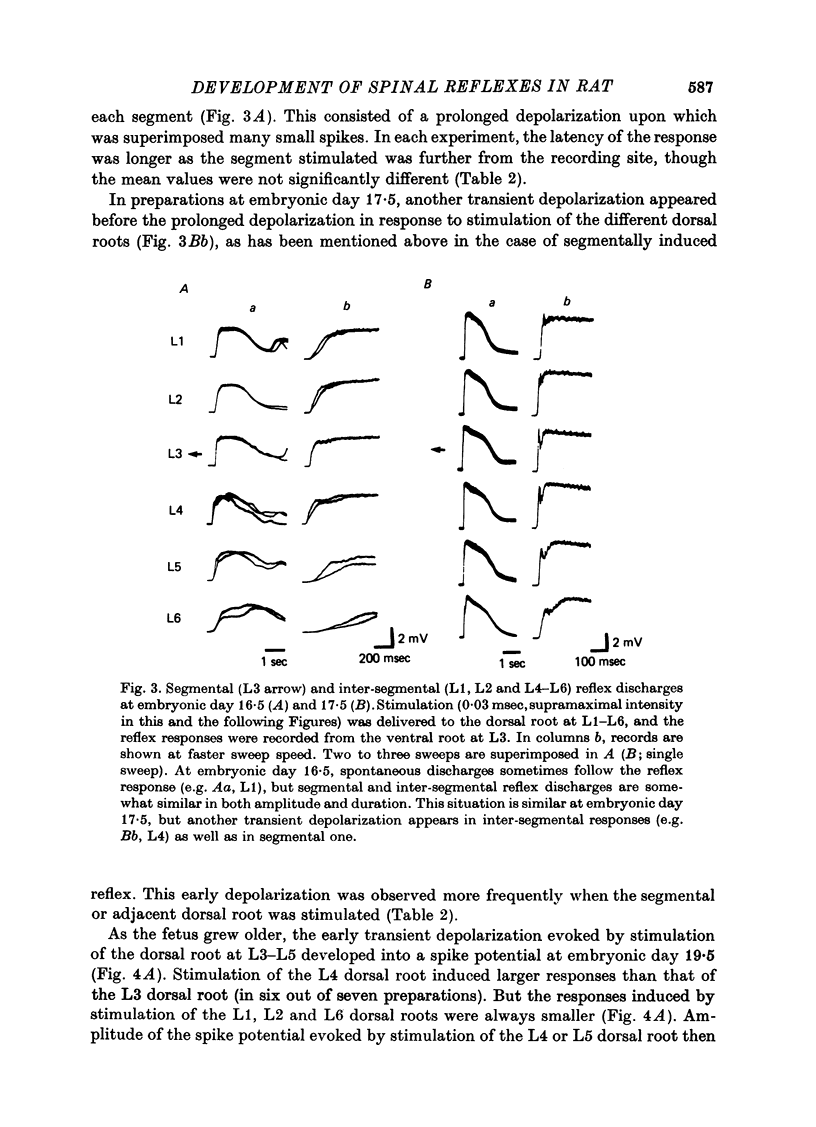
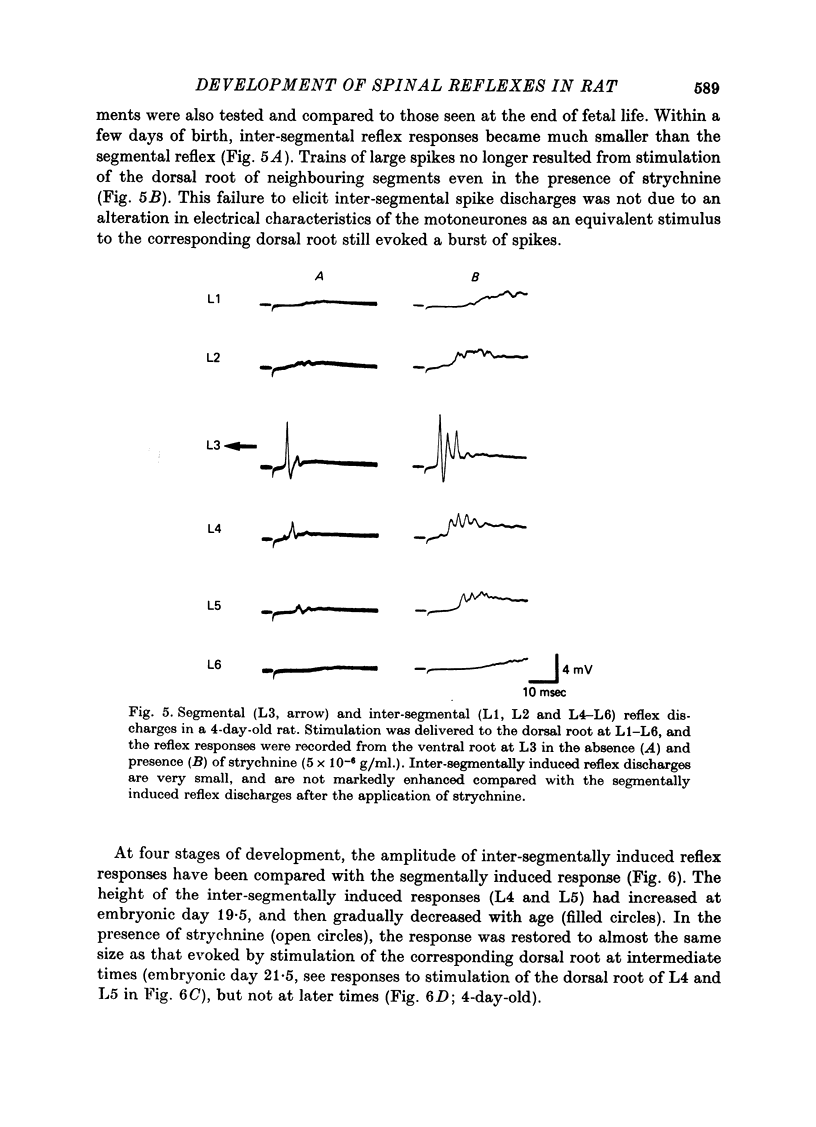
1. The onset and development of spinal reflex activity was investigated using the isolated spinal cord of the rat fetus. The potential changes generated in motoneurones were recorded extracellularly from L3 ventral roots. 2. A spike potential was recorded from the ventral root at embryonic day 13.5 in response to stimulation of the cord surface close to the ventral root. The discharge persisted in Ca2+-free solution but was blocked by tetrodotoxin. 3. At embryonic day 14.5, trans-synaptically evoked discharges were detected in motoneurones. 4. Stimulation of the dorsal root was first effective in eliciting reflex discharges at embryonic day 15.5. The reflex response then consisted of a prolonged depolarization upon which were superimposed small spikes, and was probably polysynaptic. 5. A spike potential, presumably a monosynaptic reflex, was generated at the end of fetal life. This discharge appeared first at embryonic day 17.5 in a primitive form. 6. Between embryonic day 16.5 and 17.5, stimulation of the dorsal root of diffferent segments (L1-L6) elicited responses similar to those induced by the corresponding (i.e. L3) dorsal root stimulation. These inter-segmentally induced responses were then reduced in size toward the birth. However, in the presence of strychnine, a train of spike discharges of similar shape to the segmentally induced response was also evoked by stimulation of the dorsal root at L4 or L5. These spikes disappeared during further post-natal development. 7. It is concluded that synapses in the segmental polysynaptic pathway become functional in a retrograde sequence with respect to the direction of normal reflex impulse flow. The reflex responses, elicited by stimulation of the dorsal roots of different segments, are suggested to be suppressed first by the development of inhibitory mechanisms and then by neuronal cell death or by elimination of the synapses responsible for generating the inter-segmental reflexes.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. R., Pettigrew A. G. The formation of synapses in striated muscle during development. J Physiol. 1974 Sep;241(2):515–545. doi: 10.1113/jphysiol.1974.sp010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAIN S. M., PETERSON E. R. Bioelectric activity in long-term cultures of spinal cord tissues. Science. 1963 Aug 2;141(3579):427–429. doi: 10.1126/science.141.3579.427. [DOI] [PubMed] [Google Scholar]
- CRAIN S. M., PETERSON E. R. COMPLEX BIOELECTRIC ACTIVITY IN ORGANIZED TISSUE CULTURES OF SPINAL CORD (HUMAN, RAT AND CHICK). J Cell Physiol. 1964 Aug;64:1–13. doi: 10.1002/jcp.1030640102. [DOI] [PubMed] [Google Scholar]
- Crain S. M., Peterson E. R. Onset and development of functional interneuronal connections in explants of rat spinal cord-ganglia during maturation in culture. Brain Res. 1967 Dec;6(4):750–762. doi: 10.1016/0006-8993(67)90130-8. [DOI] [PubMed] [Google Scholar]
- Crepel F., Mariani J., Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976 Nov;7(6):567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- ECCLES R. M., WILLIS W. D. THE EFFECT OF REPETITIVE STIMULATION UPON MONOSYNAPTIC TRANSMISSION IN KITTENS. J Physiol. 1965 Jan;176:311–321. doi: 10.1113/jphysiol.1965.sp007552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R. M., Shealy C. N., Willis W. D. Patterns of innervation of kitten motoneurones. J Physiol. 1963 Mar;165(3):392–402. doi: 10.1113/jphysiol.1963.sp007065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R. M., Willis W. D. Presynaptic inhibition of the monosynaptic reflex pathway in kittens. J Physiol. 1963 Mar;165(3):403–420. doi: 10.1113/jphysiol.1963.sp007066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan A. E. Differentiation and degeneration in the motor horn of the foetal mouse. J Morphol. 1969 Nov;129(3):281–305. doi: 10.1002/jmor.1051290303. [DOI] [PubMed] [Google Scholar]
- Fraher J. P. A numerical study of cervical and thoracic ventral nerve roots. J Anat. 1974 Sep;118(Pt 1):127–142. [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Kusama T. Distribution of the dorsal root fibers in the cat. An experimental study with the Nauta method. Brain Res. 1969 Apr;13(2):338–359. doi: 10.1016/0006-8993(69)90292-3. [DOI] [PubMed] [Google Scholar]
- Kellerth J. O., Mellström A., Skoglund S. Postnatal excitability changes of kitten motoneurones. Acta Physiol Scand. 1971 Sep;83(1):31–41. doi: 10.1111/j.1748-1716.1971.tb05048.x. [DOI] [PubMed] [Google Scholar]
- Konishi S., Otsuka M. Excitatory action of hypothalamic substance P on spinal motoneurones of newborn rats. Nature. 1974 Dec 20;252(5485):734–735. doi: 10.1038/252734a0. [DOI] [PubMed] [Google Scholar]
- Lichtman J. W. The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. J Physiol. 1977 Dec;273(1):155–177. doi: 10.1113/jphysiol.1977.sp012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellström A. Postnatal excitability changes of the ankle monosynaptic reflexes in the cat. Acta Physiol Scand. 1971 Aug;82(4):477–489. doi: 10.1111/j.1748-1716.1971.tb04993.x. [DOI] [PubMed] [Google Scholar]
- NAKA K. I. ELECTROPHYSIOLOGY OF THE FETAL SPINAL CORD. I. ACTION POTENTIALS OF THE MOTONEURON. J Gen Physiol. 1964 May;47:1003–1022. doi: 10.1085/jgp.47.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKA K. I. ELECTROPHYSIOLOGY OF THE FETAL SPINAL CORD. II. INTERACTION AMONG PERIPHERAL INPUTS AND RECURRENT INHIBITION. J Gen Physiol. 1964 May;47:1023–1038. doi: 10.1085/jgp.47.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M., Konishi S. Electrophysiology of mammalian spinal cord in vitro. Nature. 1974 Dec 20;252(5485):733–734. doi: 10.1038/252733a0. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Konishi S. Substance P and excitatory transmitter of primary sensory neurons. Cold Spring Harb Symp Quant Biol. 1976;40:135–143. doi: 10.1101/sqb.1976.040.01.015. [DOI] [PubMed] [Google Scholar]
- PETERSON E. R., CRAIN S. M., MURRAY M. R. DIFFERENTIATION AND PROLONGED MAINTENANCE OF BIOELECTRICALLY ACTIVE SPINAL CORD CULTURES (RAT, CHICK AND HUMAN). Z Zellforsch Mikrosk Anat. 1965 Mar 25;66(1):130–154. doi: 10.1007/BF00339322. [DOI] [PubMed] [Google Scholar]
- Provine R. R., Rogers L. Development of spinal cord bioelectric activity in spinal chick embryos and its behavioral implications. J Neurobiol. 1977 May;8(3):217–228. doi: 10.1002/neu.480080305. [DOI] [PubMed] [Google Scholar]
- Provine R. R., Sharma S. C., Sandel T. T., Hamburger V. Electrical activity in the spinal cord of the chick embryo, in situ. Proc Natl Acad Sci U S A. 1970 Mar;65(3):508–515. doi: 10.1073/pnas.65.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanes G. J. Motor localization and the effects of nerve injury on the ventral horn cells of the spinal cord. J Anat. 1946 Jul;80(Pt 3):117–131. [PMC free article] [PubMed] [Google Scholar]
- Ronnevi L. O., Conradi S. Ultrastructural evidence for spontaneous elimination of synaptic terminals on spinal motoneurons in the kitten. Brain Res. 1974 Nov 15;80(2):335–339. doi: 10.1016/0006-8993(74)90696-9. [DOI] [PubMed] [Google Scholar]
- Rosenthal J. L., Taraskevich P. S. Reduction of multiaxonal innervation at the neuromuscular junction of the rat during development. J Physiol. 1977 Sep;270(2):299–310. doi: 10.1113/jphysiol.1977.sp011953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPRAGUE J. M., HONGCHIEN H. A. THE TERMINAL FIELDS OF DORSAL ROOT FIBERS IN THE LUMBOSACRAL SPINAL CORD OF THE CAT, AND THE DENDRITIC ORGANIZATION OF THE MOTOR NUCLEI. Prog Brain Res. 1964;11:120–154. doi: 10.1016/s0079-6123(08)64046-7. [DOI] [PubMed] [Google Scholar]
- SPRAGUE J. M. The distribution of dorsal root fibres on motor cells in the lumbosacral spinal cord of the cat, and the site of excitatory and inhibitory terminals in monosynaptic pathways. Proc R Soc Lond B Biol Sci. 1958 Dec 24;149(937):534–556. doi: 10.1098/rspb.1958.0091. [DOI] [PubMed] [Google Scholar]
- Sharma S. C., Provine R. R., Hamburger V., Sandel T. T. Unit activity in the isolated spinal cord of chick embryo, in situ. Proc Natl Acad Sci U S A. 1970 May;66(1):40–47. doi: 10.1073/pnas.66.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. E., Grieshaber J. A. A morphological investigation of an early reflex pathway in developing rat spinal cord. J Comp Neurol. 1973 Mar 15;148(2):177–209. doi: 10.1002/cne.901480205. [DOI] [PubMed] [Google Scholar]
- WILSON V. J. Reflex transmission in the kitten. J Neurophysiol. 1962 Mar;25:263–276. doi: 10.1152/jn.1962.25.2.263. [DOI] [PubMed] [Google Scholar]


