Abstract
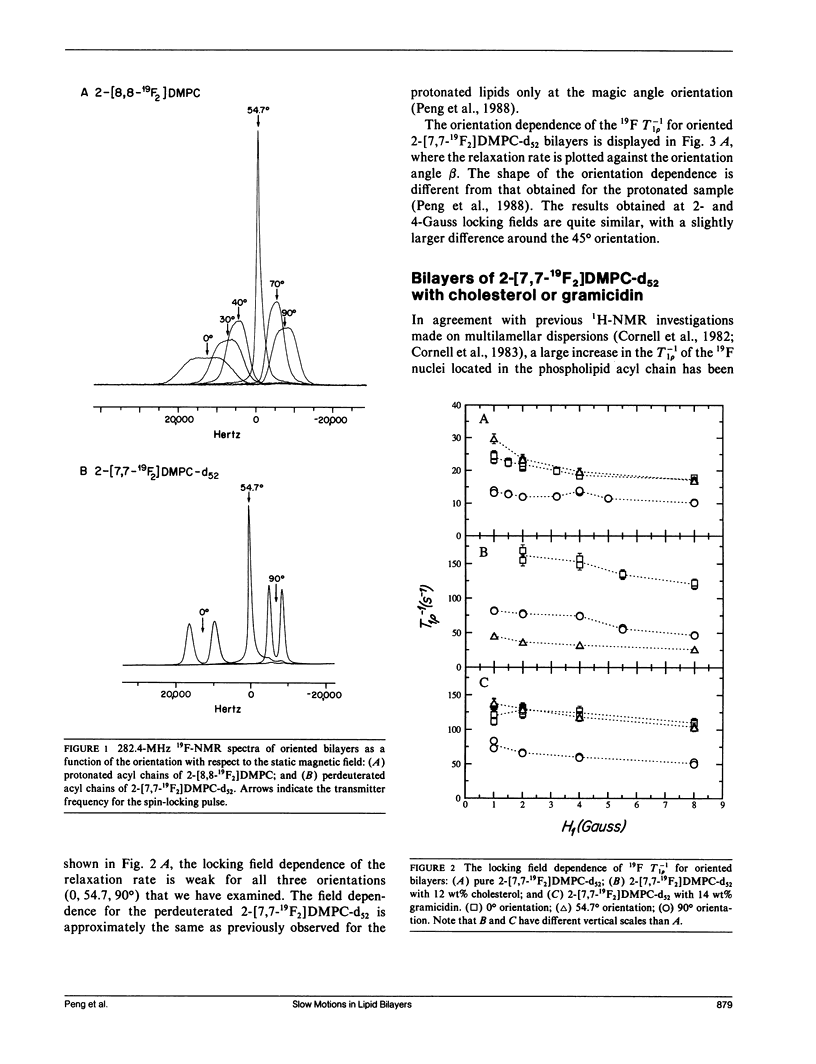
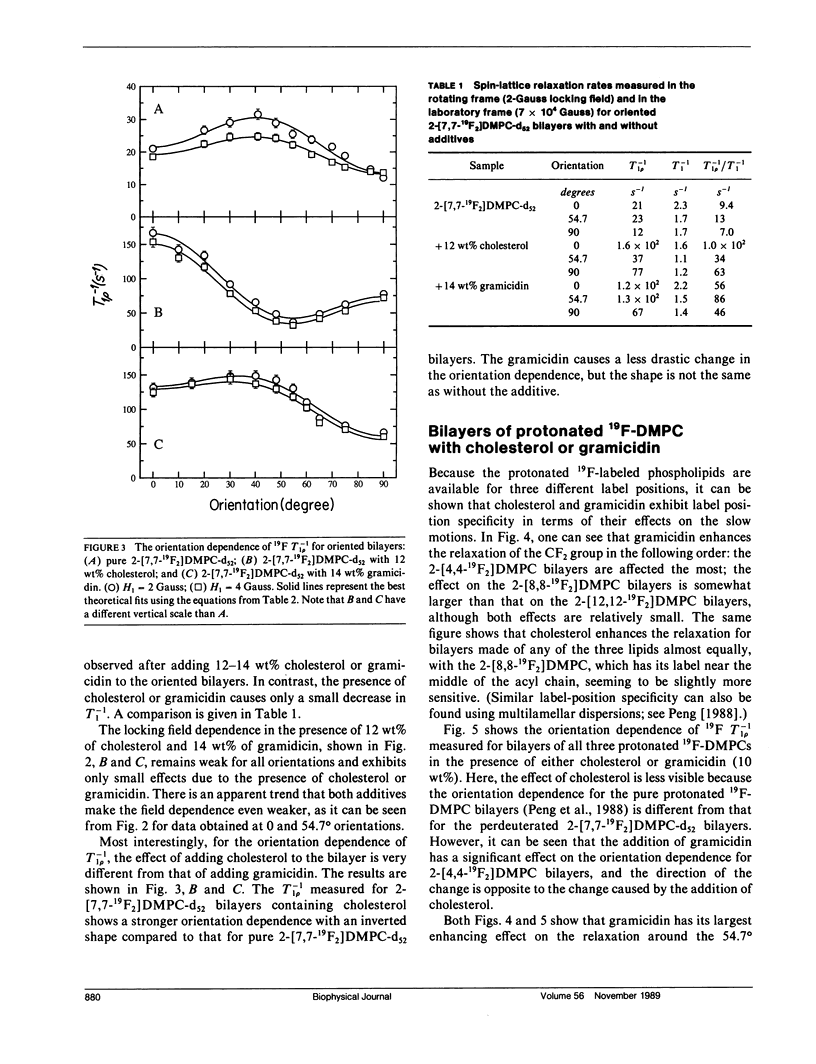
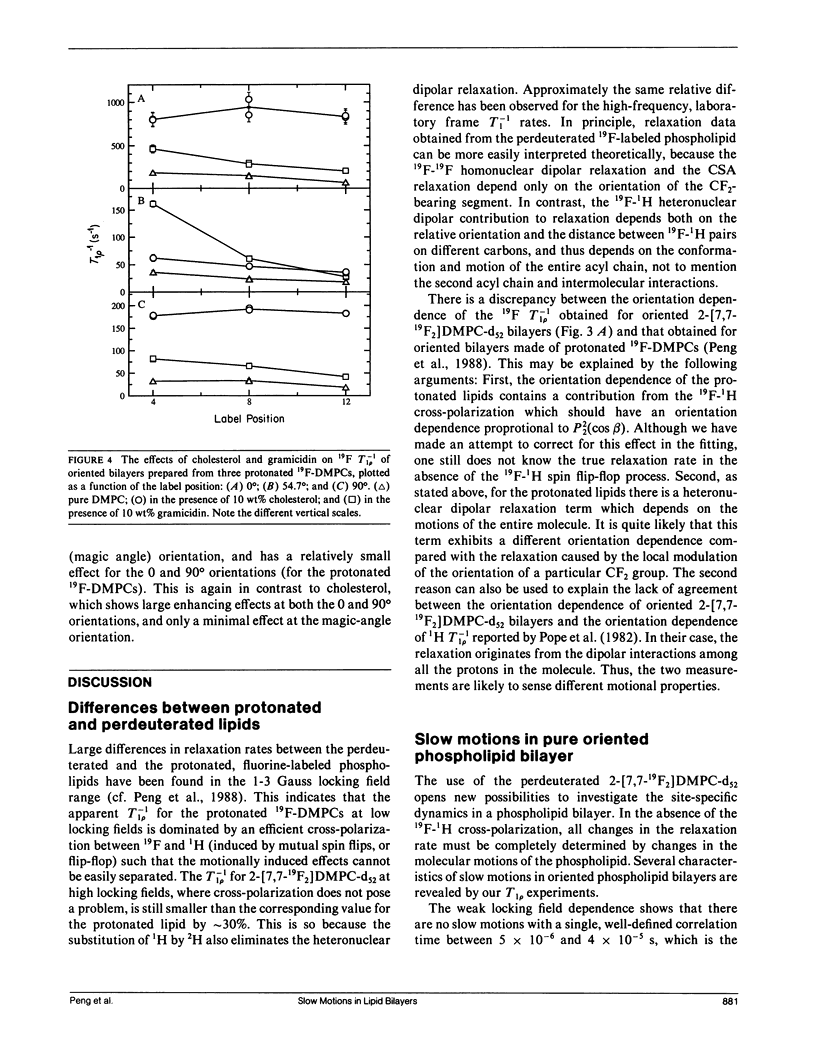
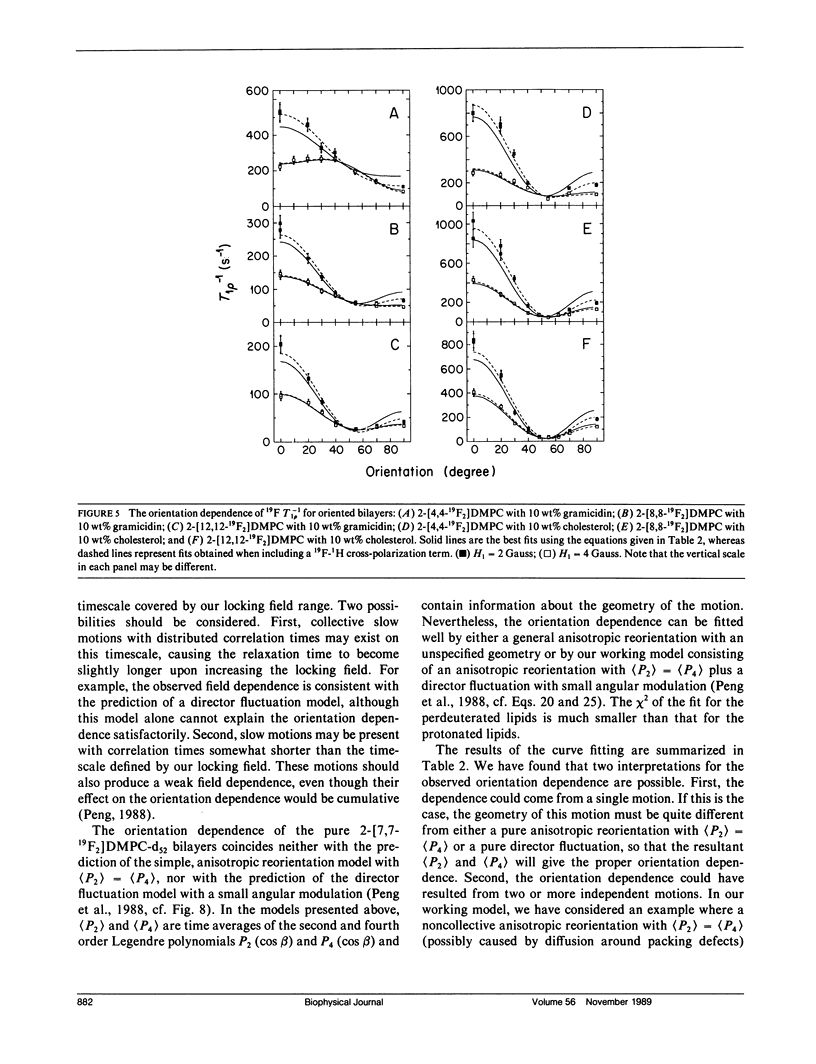
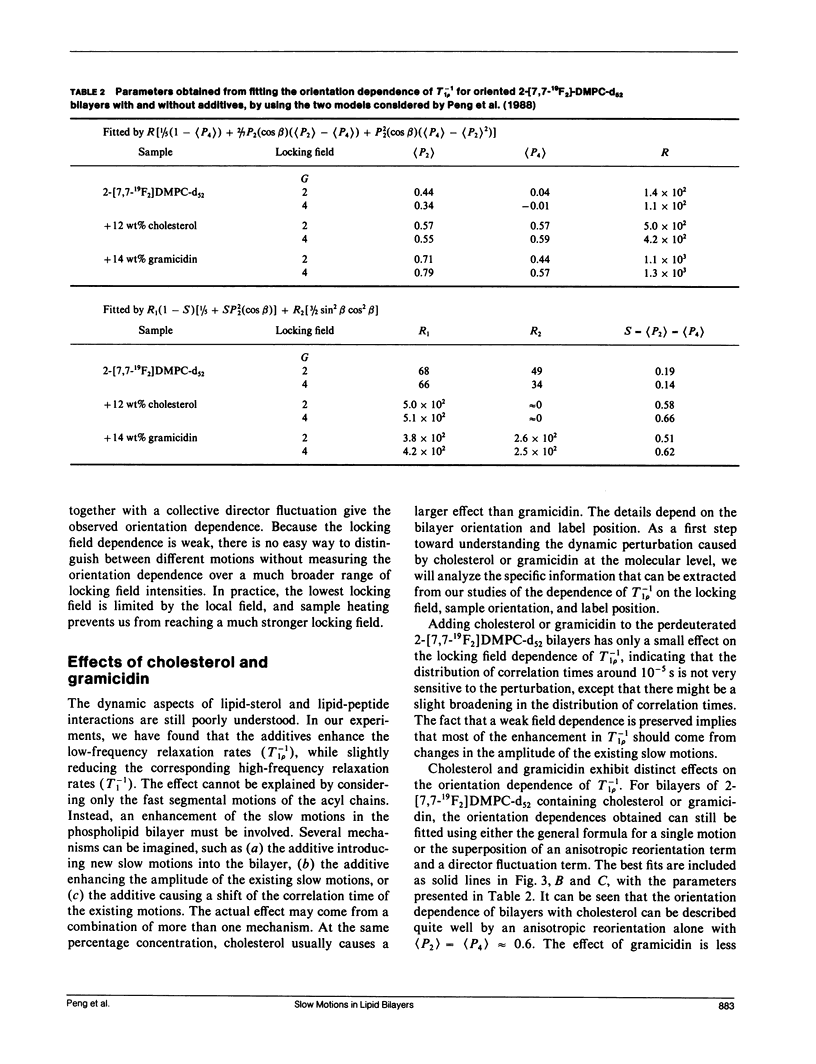
In an extension of our earlier work (Peng, Z.-y., V. Simplaceanu, I. J. Lowe, and C. Ho. 1988. Biophys. J. 54:81-95), the rotating-frame nuclear spin-lattice relaxation (T1 rho) technique has been used to investigate the slow molecular motions (10(-4) - 10(-6) s) in lipid bilayers prepared from protonated or perdeuterated 19F-labeled phospholipids in the absence and presence of cholesterol or gramicidin as membrane-interacting molecules. Complications caused by the 19F-1H cross-polarization observed previously can be removed by the substitution of 2H for 1H in the acyl chains. Only a weak dependence of the T-1(1 rho) on the locking field strength is found for a phospholipid molecule with perdeuterated acyl chains, indicating that there are no slow motions with a single, well-defined correlation time between 5 x 10(-6) and 4 x 10(-5) s. However, the orientation dependences of the T-1(1 rho) can be well fitted by motional models with either one slow motion having an unspecified geometry or with a superposition of two specific types of slow motions. Cholesterol and gramicidin show distinct effects in altering either the geometry or the weighting of slow motions in phospholipid bilayers, as reflected by changes in the orientation dependence. These two additives also exhibit quite different label-position specificities. A qualitative understanding of the induced effects of cholesterol and gramicidin on the dynamics of phospholipid bilayers will be discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S. Gramicidin channels. Annu Rev Physiol. 1984;46:531–548. doi: 10.1146/annurev.ph.46.030184.002531. [DOI] [PubMed] [Google Scholar]
- Brown M. F., Deese A. J., Dratz E. A. Proton, carbon-13, and phosphorus-31 NMR methods for the investigation of rhodopsin--lipid interactions in retinal rod outer segment membranes. Methods Enzymol. 1982;81:709–728. doi: 10.1016/s0076-6879(82)81098-7. [DOI] [PubMed] [Google Scholar]
- Chupin V., Killian J. A., de Kruijff B. 2H-nuclear magnetic resonance investigations on phospholipid acyl chain order and dynamics in the gramicidin-induced hexagonal HII phase. Biophys J. 1987 Mar;51(3):395–405. doi: 10.1016/S0006-3495(87)83361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell B. A., Davenport J. B., Separovic F. Low-frequency motion in membranes. The effect of cholesterol and proteins. Biochim Biophys Acta. 1982 Jul 28;689(2):337–345. doi: 10.1016/0005-2736(82)90267-x. [DOI] [PubMed] [Google Scholar]
- Cornell B. A., Hiller R. G., Raison J., Separovic F., Smith R., Vary J. C., Morris C. Biological membranes are rich in low-frequency motion. Biochim Biophys Acta. 1983 Jul 27;732(2):473–478. doi: 10.1016/0005-2736(83)90065-2. [DOI] [PubMed] [Google Scholar]
- Cornell B. Gramicidin A--phospholipid model systems. J Bioenerg Biomembr. 1987 Dec;19(6):655–676. doi: 10.1007/BF00762301. [DOI] [PubMed] [Google Scholar]
- Engelsberg M., Dowd S. R., Simplaceanu V., Cook B. W., Ho C. Nuclear magnetic resonance line-shape analysis of fluorine-19-labeled phospholipids. Biochemistry. 1982 Dec 21;21(26):6985–6989. doi: 10.1021/bi00269a056. [DOI] [PubMed] [Google Scholar]
- Huang C. H. A structural model for the cholesterol-phosphatidylcholine complexes in bilayer membranes. Lipids. 1977 Apr;12(4):348–356. doi: 10.1007/BF02533637. [DOI] [PubMed] [Google Scholar]
- Jacobs R., Oldfield E. Deuterium nuclear magnetic resonance investigation of dimyristoyllecithin--dipalmitoyllecithin and dimyristoyllecithin--cholesterol mixtures. Biochemistry. 1979 Jul 24;18(15):3280–3285. doi: 10.1021/bi00582a013. [DOI] [PubMed] [Google Scholar]
- Peng Z. Y., Simplaceanu V., Lowe I. J., Ho C. Rotating-frame relaxation studies of slow motions in fluorinated phospholipid model membranes. Biophys J. 1988 Jul;54(1):81–95. doi: 10.1016/S0006-3495(88)82933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separations in binary mixtures of cholesterol and phospholipids. Biochem Biophys Res Commun. 1973 Jul 17;53(2):446–451. doi: 10.1016/0006-291x(73)90682-7. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Smith R. L., Oldfield E. Dynamic structure of membranes by deuterium NMR. Science. 1984 Jul 20;225(4659):280–288. doi: 10.1126/science.6740310. [DOI] [PubMed] [Google Scholar]
- Tamm L. K., Seelig J. Lipid solvation of cytochrome c oxidase. Deuterium, nitrogen-14, and phosphorus-31 nuclear magnetic resonance studies on the phosphocholine head group and on cis-unsaturated fatty acyl chains. Biochemistry. 1983 Mar 15;22(6):1474–1483. doi: 10.1021/bi00275a023. [DOI] [PubMed] [Google Scholar]
- Weinstein S., Wallace B. A., Blout E. R., Morrow J. S., Veatch W. Conformation of gramicidin A channel in phospholipid vesicles: a 13C and 19F nuclear magnetic resonance study. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4230–4234. doi: 10.1073/pnas.76.9.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeagle P. L. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985 Dec 9;822(3-4):267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]


