Abstract
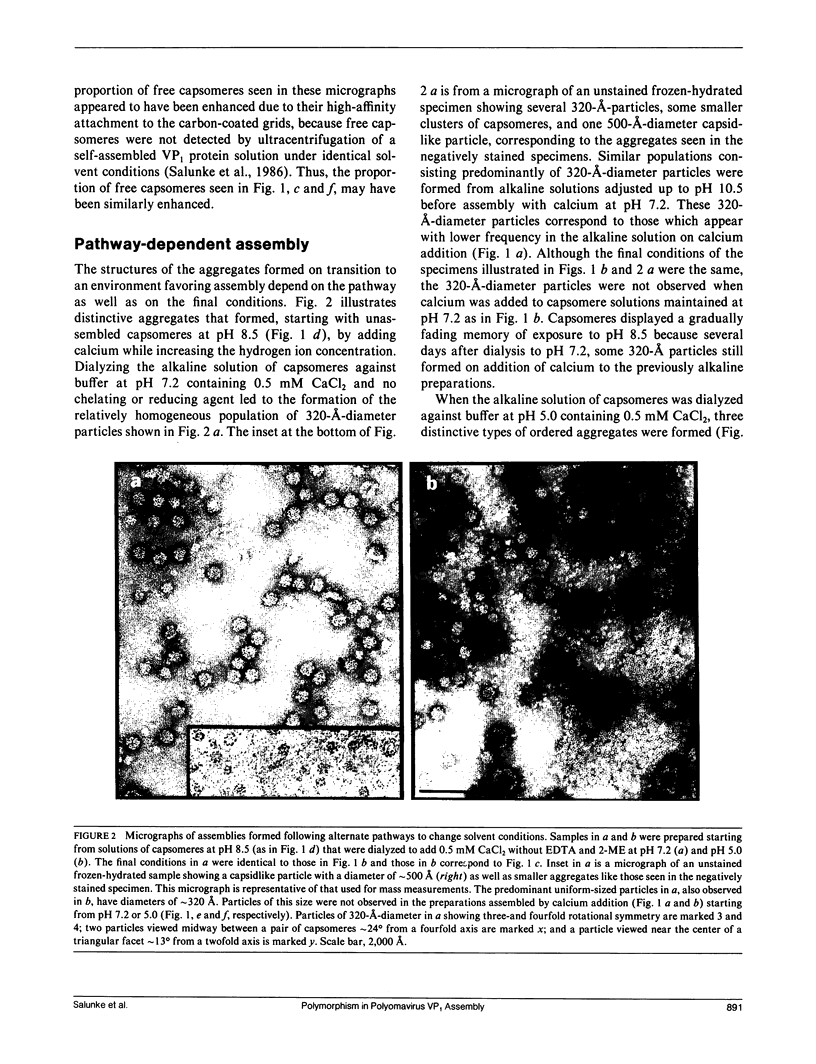
Polyomavirus major capsid protein VP1, purified after expression of the recombinant gene in Escherichia coli, forms stable pentamers in low-ionic strength, neutral, or alkaline solutions. Electron microscopy showed that the pentamers, which correspond to viral capsomeres, can be self-assembled into a variety of polymorphic aggregates by lowering the pH, adding calcium, or raising the ionic strength. Some of the aggregates resembled the 500-A-diameter virus capsid, whereas other considerably larger or smaller capsids were also produced. The particular structures formed on transition to an environment favoring assembly depended on the pathway of the solvent changes as well as on the final conditions. Mass measurements from cryoelectron micrographs and image analysis of negatively stained specimens established that a distinctive 320-A-diameter particle consists of 24 close-packed pentamers arranged with octahedral symmetry. Comparison of this unexpected octahedral assembly with a 12-capsomere icosahedral aggregate and the 72-capsomere icosahedral virus capsid by computer graphics methods indicates that similar connections are made among trimers of pentamers in these shells of different size. The polymorphism in the assembly of VP1 pentamers can be related to the switching in bonding specificity required to build the virus capsid.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akervall K., Strandberg B., Rossmann M. G., Bengtsson U., Fridborg K., Johannisen H., Kannan K. K., Lövgren S., Petef G., Oberg B. X-ray diffraction studies of the structure of satellite tobacco necrosis virus. Cold Spring Harb Symp Quant Biol. 1972;36:469–483. doi: 10.1101/sqb.1972.036.01.059. [DOI] [PubMed] [Google Scholar]
- Anderer F. A., Schlumberger H. D., Koch M. A., Frank H., Eggers H. J. Structure of simian virus 40. II. Symmetry and components of the virus particle. Virology. 1967 Jul;32(3):511–523. doi: 10.1016/0042-6822(67)90303-0. [DOI] [PubMed] [Google Scholar]
- Baker T. S., Caspar D. L., Murakami W. T. Polyoma virus 'hexamer' tubes consist of paired pentamers. Nature. 1983 Jun 2;303(5916):446–448. doi: 10.1038/303446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft J. B. The self-assembly of spherical plant viruses. Adv Virus Res. 1970;16:99–134. doi: 10.1016/s0065-3527(08)60022-6. [DOI] [PubMed] [Google Scholar]
- Brady J. N., Winston V. D., Consigli R. A. Dissociation of polyoma virus by the chelation of calcium ions found associated with purified virions. J Virol. 1977 Sep;23(3):717–724. doi: 10.1128/jvi.23.3.717-724.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASPAR D. L. ASSEMBLY AND STABILITY OF THE TOBACCO MOSAIC VIRUS PARTICLE. Adv Protein Chem. 1963;18:37–121. doi: 10.1016/s0065-3233(08)60268-5. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- CRICK F. H., WATSON J. D. Structure of small viruses. Nature. 1956 Mar 10;177(4506):473–475. doi: 10.1038/177473a0. [DOI] [PubMed] [Google Scholar]
- Christiansen G., Landers T., Griffith J., Berg P. Characterization of components released by alkali disruption of simian virus 40. J Virol. 1977 Mar;21(3):1079–1084. doi: 10.1128/jvi.21.3.1079-1084.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther R. A., Amos L. A. Harmonic analysis of electron microscope images with rotational symmetry. J Mol Biol. 1971 Aug 28;60(1):123–130. doi: 10.1016/0022-2836(71)90452-9. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Adrian M., Chang J. J., Homo J. C., Lepault J., McDowall A. W., Schultz P. Cryo-electron microscopy of vitrified specimens. Q Rev Biophys. 1988 May;21(2):129–228. doi: 10.1017/s0033583500004297. [DOI] [PubMed] [Google Scholar]
- Durham A. C., Hendry D. A., Von Wechmar M. B. Does calcium ion binding control plant virus disassembly? Virology. 1977 Apr;77(2):524–533. doi: 10.1016/0042-6822(77)90478-0. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Klug A. The structure of viruses of the papilloma-polyoma type 3. Structure of rabbit papilloma virus, with an appendix on the topography of contrast in negative-staining for electron-microscopy. J Mol Biol. 1965 Aug;13(1):1–12. doi: 10.1016/s0022-2836(65)80075-4. [DOI] [PubMed] [Google Scholar]
- Friedmann T., David D. Structural roles of polyoma virus proteins. J Virol. 1972 Oct;10(4):776–782. doi: 10.1128/jvi.10.4.776-782.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea R. L., Salunke D. M., Caspar D. L. Site-directed mutation affecting polyomavirus capsid self-assembly in vitro. Nature. 1987 Sep 3;329(6134):86–87. doi: 10.1038/329086a0. [DOI] [PubMed] [Google Scholar]
- Harrison S. C. Protein interfaces and intersubunit bonding. The case of tomato bushy stunt virus. Biophys J. 1980 Oct;32(1):139–153. doi: 10.1016/S0006-3495(80)84930-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLUG A. STRUCTURE OF VIRUSES OF THE PAPILLOMA-POLYOMA TYPE. II. COMMENTS ON OTHER WORK. J Mol Biol. 1965 Feb;11:424–431. doi: 10.1016/s0022-2836(65)80067-5. [DOI] [PubMed] [Google Scholar]
- Kiselev N. A., Klug A. The structure of viruses of the papilloma-polyoma type. V. Tubular variants built of pentamers. J Mol Biol. 1969 Mar 14;40(2):155–171. doi: 10.1016/0022-2836(69)90465-3. [DOI] [PubMed] [Google Scholar]
- Klug A. Interpretation of the rotation function map of satellite tobacco necrosis virus: octahedral packing of icosahedral particles. Cold Spring Harb Symp Quant Biol. 1972;36:483–487. doi: 10.1101/sqb.1972.036.01.060. [DOI] [PubMed] [Google Scholar]
- Koch M. A., Eggers H. J., Anderer F. A., Schlumberger H. D., Frank H. Structure of simian virus 40. I. Purification and physical characterization of the virus particle. Virology. 1967 Jul;32(3):503–510. doi: 10.1016/0042-6822(67)90302-9. [DOI] [PubMed] [Google Scholar]
- Leavitt A. D., Roberts T. M., Garcea R. L. Polyoma virus major capsid protein, VP1. Purification after high level expression in Escherichia coli. J Biol Chem. 1985 Oct 15;260(23):12803–12809. [PubMed] [Google Scholar]
- Mattern C. F., Takemoto K. K., DeLeva A. M. Electron microscopic observations on multiple polyoma virus-related particles. Virology. 1967 Jul;32(3):378–392. doi: 10.1016/0042-6822(67)90288-7. [DOI] [PubMed] [Google Scholar]
- Namba K., Caspar D. L., Stubbs G. Enhancement and simplification of macromolecular images. Biophys J. 1988 Apr;53(4):469–475. doi: 10.1016/S0006-3495(88)83125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba K., Stubbs G. Structure of tobacco mosaic virus at 3.6 A resolution: implications for assembly. Science. 1986 Mar 21;231(4744):1401–1406. doi: 10.1126/science.3952490. [DOI] [PubMed] [Google Scholar]
- Okada Y. Molecular assembly of tobacco mosaic virus in vitro. Adv Biophys. 1986;22:95–149. doi: 10.1016/0065-227x(86)90004-3. [DOI] [PubMed] [Google Scholar]
- Rayment I., Baker T. S., Caspar D. L., Murakami W. T. Polyoma virus capsid structure at 22.5 A resolution. Nature. 1982 Jan 14;295(5845):110–115. doi: 10.1038/295110a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Abad-Zapatero C., Hermodson M. A., Erickson J. W. Subunit interactions in southern bean mosaic virus. J Mol Biol. 1983 May 5;166(1):37–73. doi: 10.1016/s0022-2836(83)80049-7. [DOI] [PubMed] [Google Scholar]
- Salunke D. M., Caspar D. L., Garcea R. L. Self-assembly of purified polyomavirus capsid protein VP1. Cell. 1986 Sep 12;46(6):895–904. doi: 10.1016/0092-8674(86)90071-1. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Griffin B. E. Polyoma virus DNA: complete nucleotide sequence of the gene which codes for polyoma virus capsid protein VP1 and overlaps the VP2/VP3 genes. J Virol. 1980 Feb;33(2):619–630. doi: 10.1128/jvi.33.2.619-630.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne H. V., House W., Kisch A. L. Electrophoretic properties and purification of large and small plaque-forming strains of polyoma virus. Virology. 1965 Sep;27(1):37–43. doi: 10.1016/0042-6822(65)90141-8. [DOI] [PubMed] [Google Scholar]
- Walter G., Deppert W. Intermolecular disulfide bonds: an important structural feature of the polyoma virus capsid. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):255–257. doi: 10.1101/sqb.1974.039.01.033. [DOI] [PubMed] [Google Scholar]
- ZEITLER E., BAHR G. F. Contributions to the quantitative interpretation of electron microscope pictures. Exp Cell Res. 1957 Feb;12(1):45–65. doi: 10.1016/0014-4827(57)90293-8. [DOI] [PubMed] [Google Scholar]








