Abstract
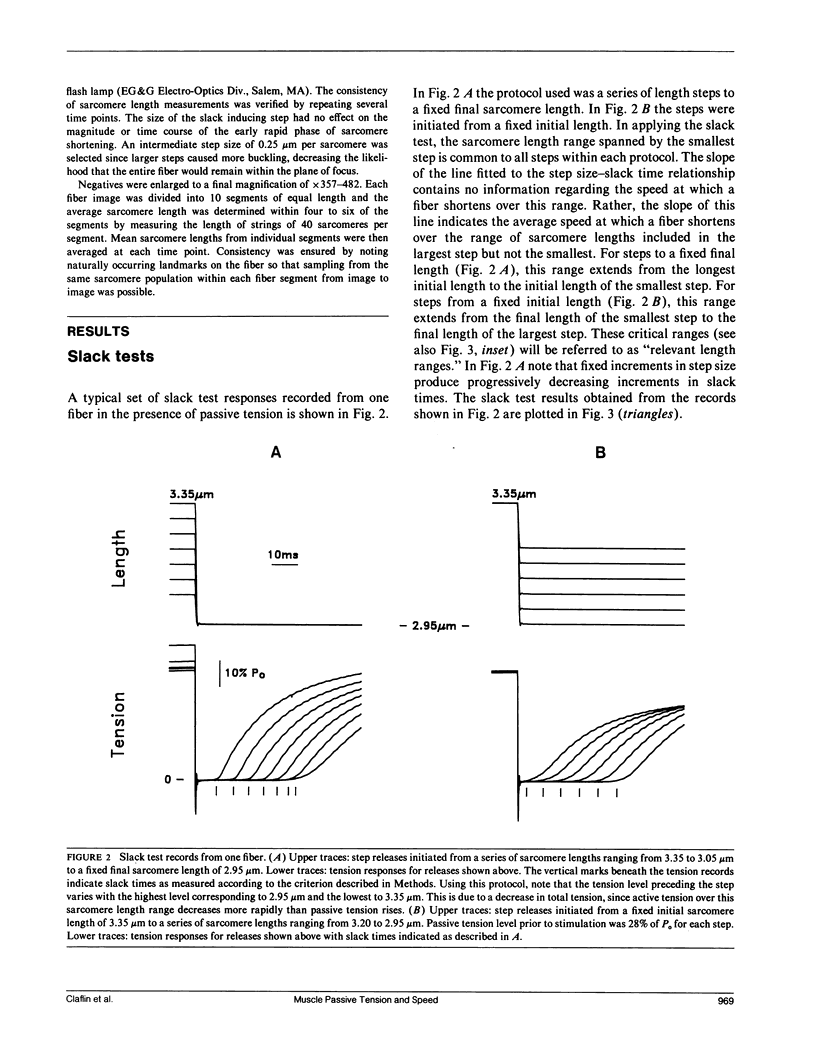
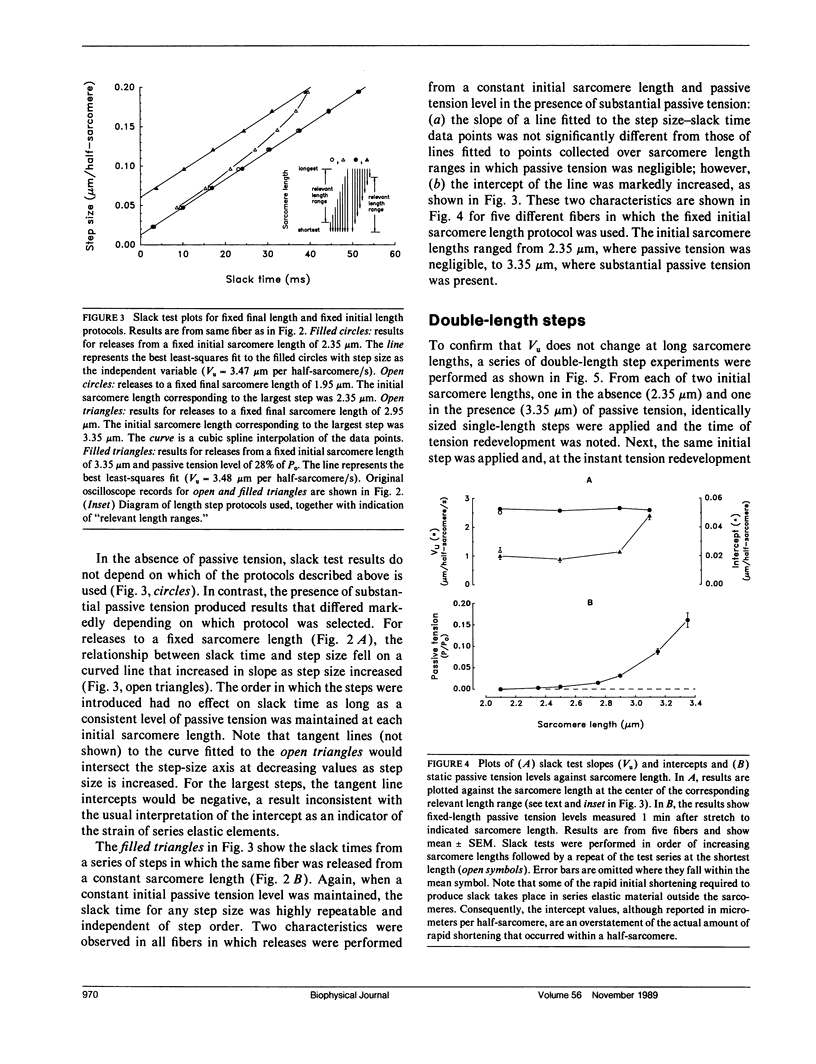
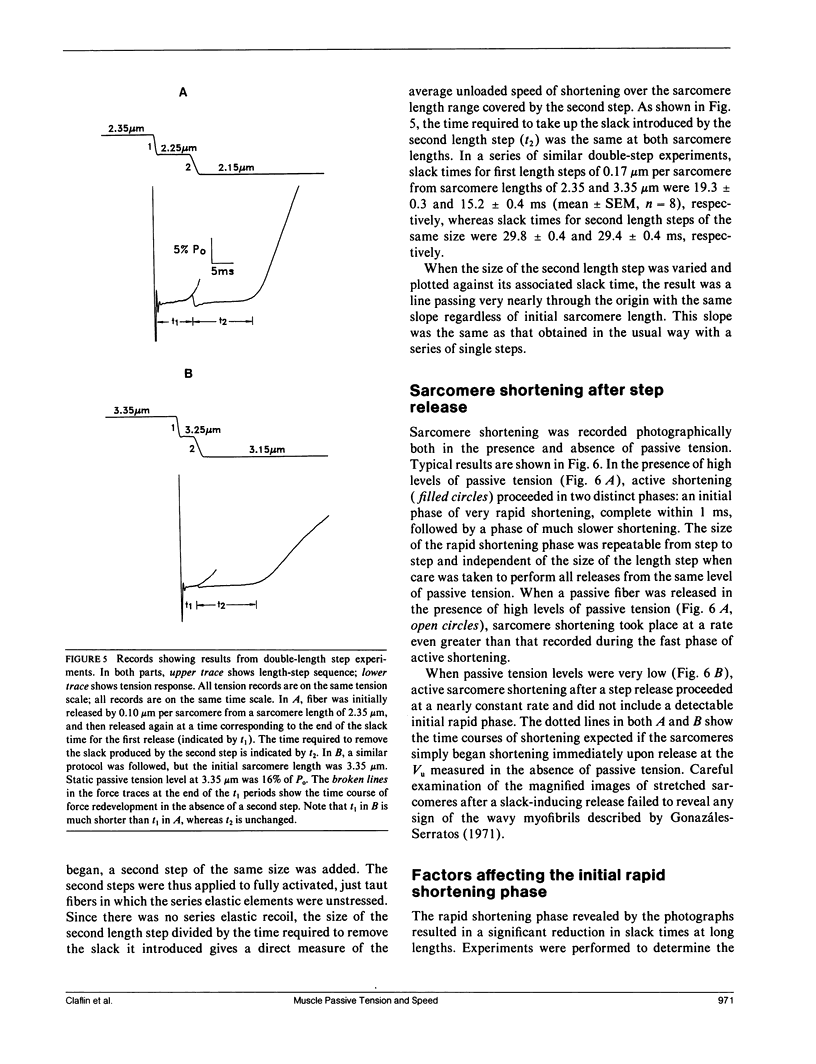
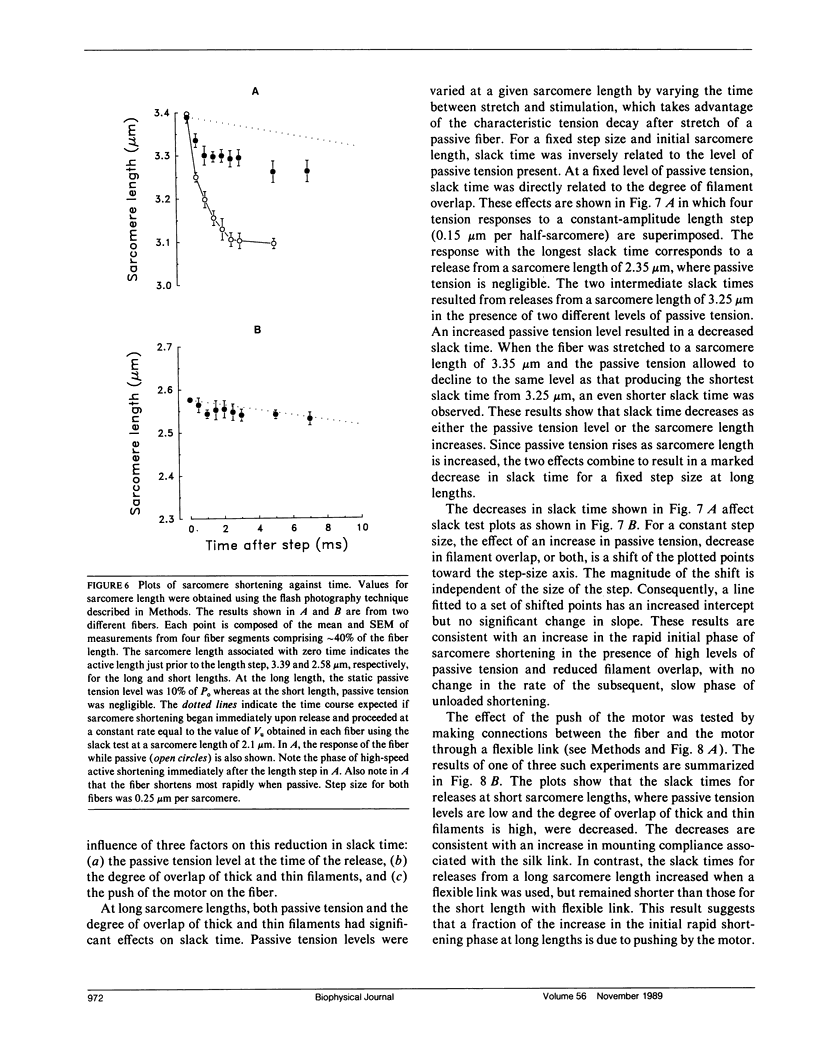
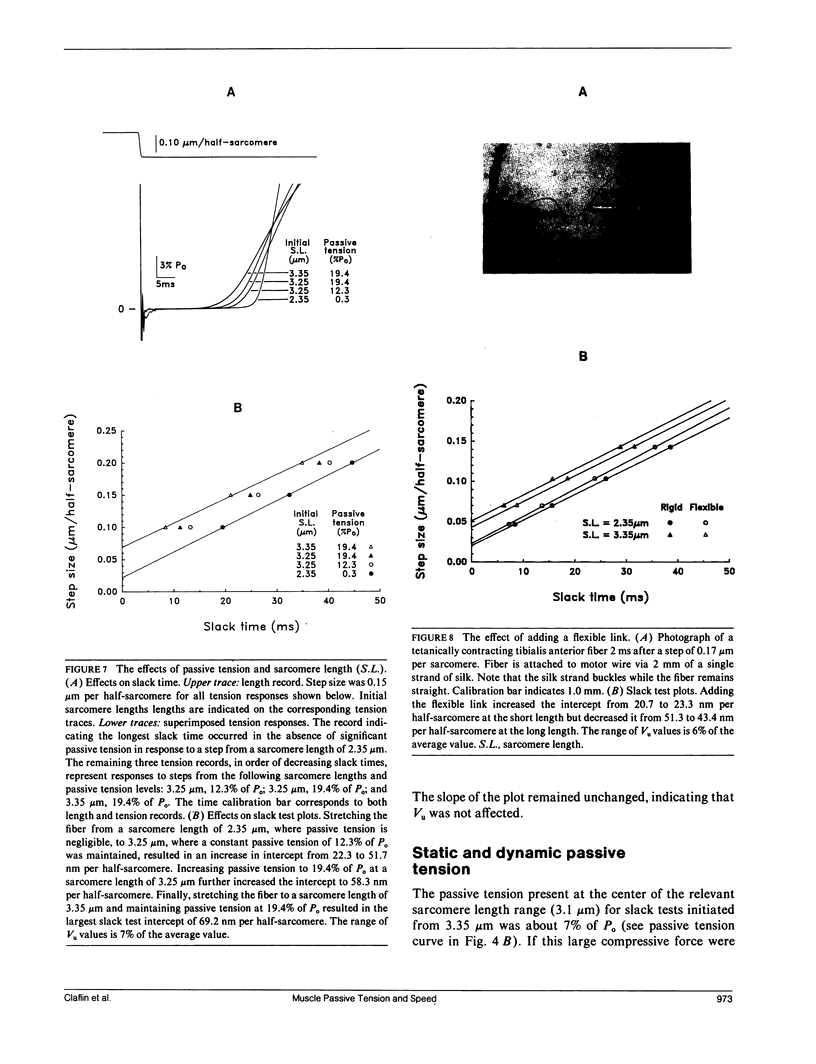
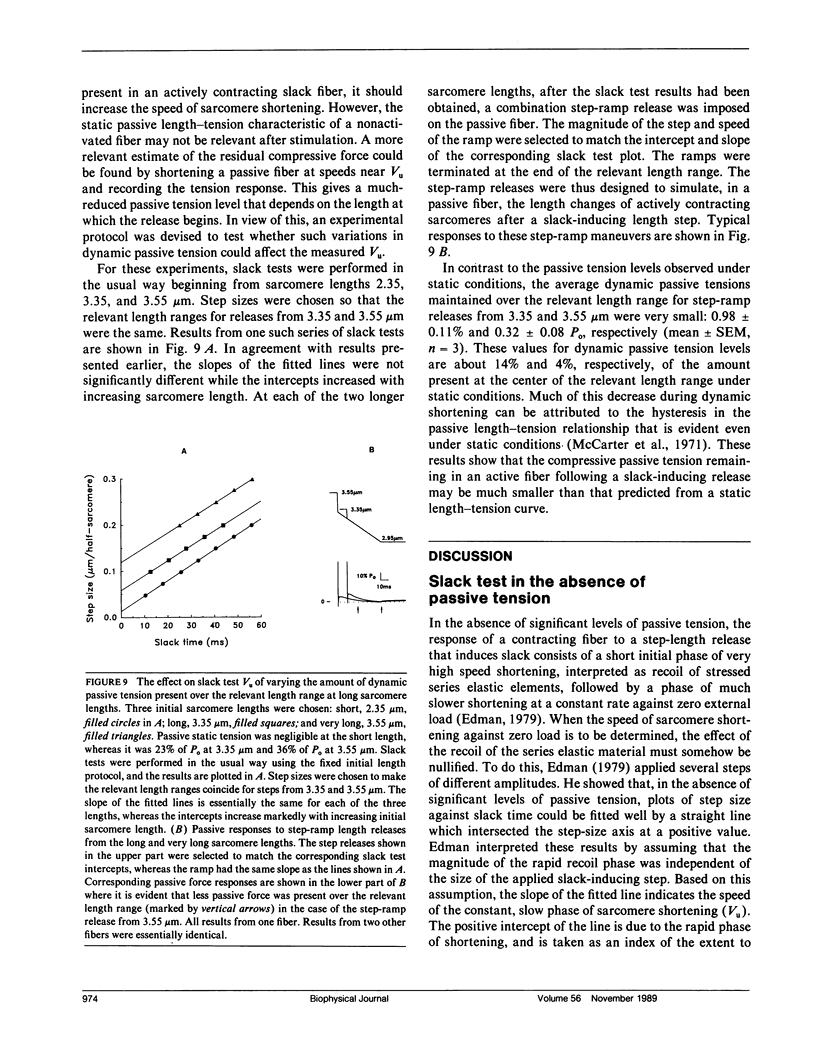
Experiments were performed to determine the influence of sarcomere length and passive tension on the velocity of unloaded shortening (Vu) as measured by the slack test technique. Slack test results were obtained from intact twitch fibers isolated from the frog (Rana temporaria). Measurements were made both in the absence and presence of passive tension using two different protocols. In one, all releases were initiated from the same sarcomere length and passive tension level; in the other, all releases ended at the same sarcomere length. In the absence of passive tension, no difference was observed between the results from the two slack test protocols. When passive tension was present, performing all releases from the same initial sarcomere length and passive tension level resulted in linear step size-slack time relationships in which the slopes (Vu) were independent of length over a sarcomere length range extending to 3.1 microns, and the intercepts increased with increasing sarcomere length. Performing all releases to the same final sarcomere length in the presence of passive tension produced nonlinear step size-slack time relationships. The results presented here show that, in the presence of significant levels of passive tension, the traditional interpretation of the slope of the slack test plot as the constant unloaded shortening velocity is only correct when all length steps are initiated from the same initial sarcomere length and level of passive tension.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edman K. A. Double-hyperbolic force-velocity relation in frog muscle fibres. J Physiol. 1988 Oct;404:301–321. doi: 10.1113/jphysiol.1988.sp017291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Serratos H. Inward spread of activation in vertebrate muscle fibres. J Physiol. 1971 Feb;212(3):777–799. doi: 10.1113/jphysiol.1971.sp009356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY H. E. X-ray analysis and the problem of muscle. Proc R Soc Lond B Biol Sci. 1953 Mar 11;141(902):59–62. doi: 10.1098/rspb.1953.0017. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Morgan D. L. Intersarcomere dynamics during fixed-end tetanic contractions of frog muscle fibres. J Physiol. 1979 Aug;293:365–378. doi: 10.1113/jphysiol.1979.sp012894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Rome L. C., Stephenson D. G., Striz S. The maximum speed of shortening in living and skinned frog muscle fibres. J Physiol. 1986 Jan;370:181–199. doi: 10.1113/jphysiol.1986.sp015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter R. J., Nabarro F. R., Wyndham C. H. Reversibility of the passive length-tension relation in mammalian skeletal muscle. Arch Int Physiol Biochim. 1971 Aug;79(3):469–479. doi: 10.3109/13813457109085331. [DOI] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Shortening velocity in skinned single muscle fibers. Influence of filament lattice spacing. Biophys J. 1987 Jul;52(1):127–131. doi: 10.1016/S0006-3495(87)83197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T. Passive interaction between sliding filaments in the osmotically compressed skinned muscle fibers of the frog. Biophys J. 1988 Mar;53(3):415–423. doi: 10.1016/S0006-3495(88)83118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]



