Abstract
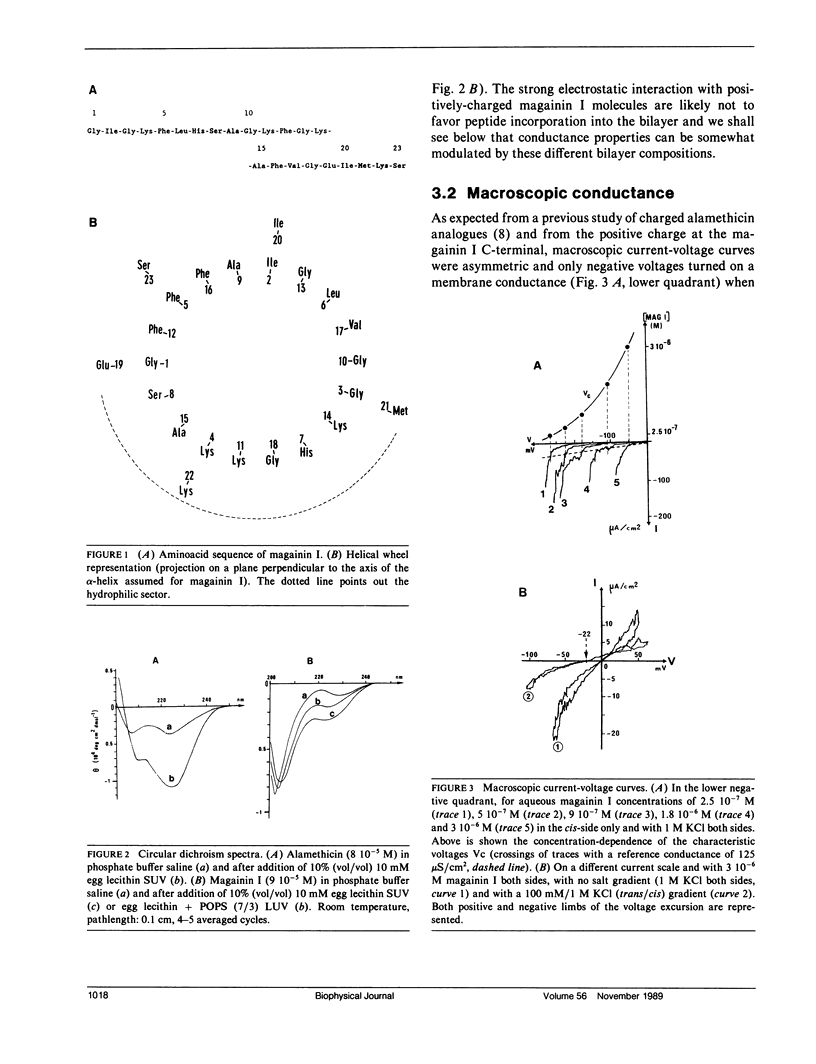
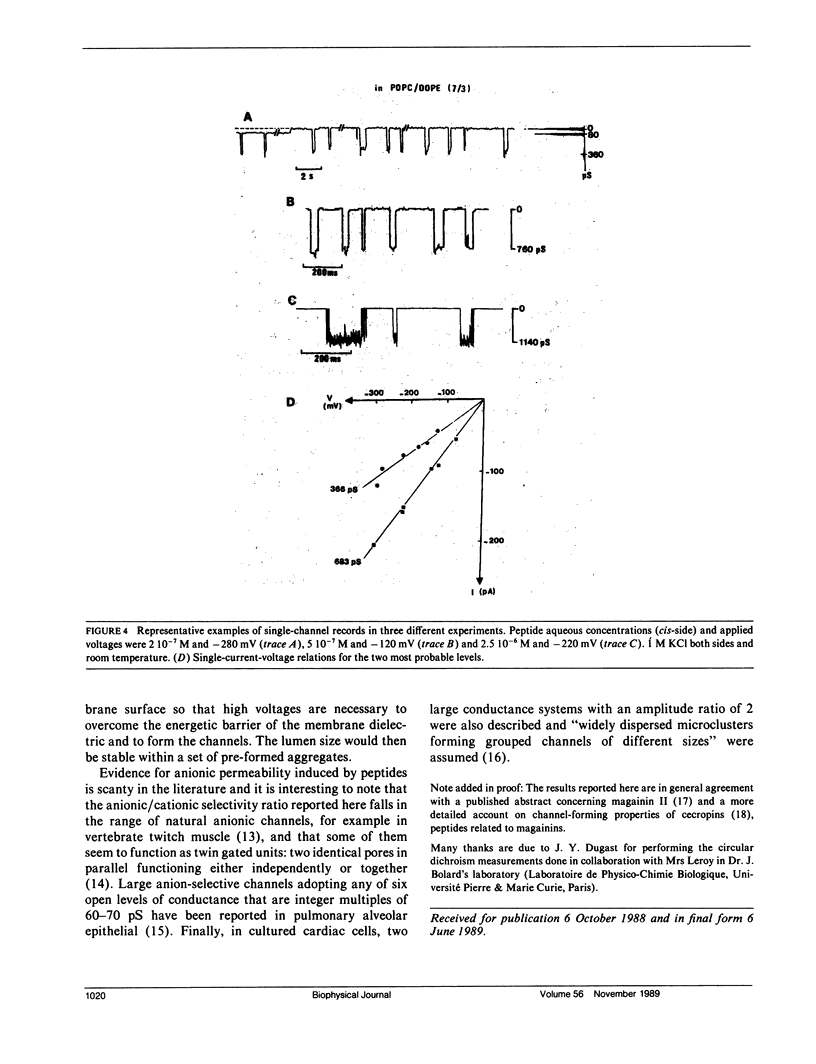
The ionophore properties of magainin I, an antimicrobial and amphipathic peptide from the skin of Xenopus, were investigated in planar lipid bilayers. Circular dichroism studies, performed comparatively with alamethicin, in small or large unilamellar phospholipidic vesicles, point to a smaller proportion of alpha-helical conformation in membranes. A weakly voltage-dependent macroscopic conductance which is anion-selective is developed when using large aqueous peptide concentration with lipid bilayer under high voltages. Single-channel experiments revealed two main conductance levels occurring independently in separate trials. Pre-aggregates lying on the membrane surface at rest and drawn into the bilayer upon voltage application are assumed to account for this behaviour contrasting with the classical multistates displayed by alamethicin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernheimer A. W., Rudy B. Interactions between membranes and cytolytic peptides. Biochim Biophys Acta. 1986 Jun 12;864(1):123–141. doi: 10.1016/0304-4157(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Boheim G. Statistical analysis of alamethicin channels in black lipid membranes. J Membr Biol. 1974;19(3):277–303. doi: 10.1007/BF01869983. [DOI] [PubMed] [Google Scholar]
- Christensen B., Fink J., Merrifield R. B., Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5072–5076. doi: 10.1073/pnas.85.14.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe A., Duclohier H., Coraboeuf E., Touzet N. Single chloride-permeable channels of large conductance in cultured cardiac cells of new-born rats. Eur Biophys J. 1987;14(3):155–162. doi: 10.1007/BF00253840. [DOI] [PubMed] [Google Scholar]
- Fox R. O., Jr, Richards F. M. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution. Nature. 1982 Nov 25;300(5890):325–330. doi: 10.1038/300325a0. [DOI] [PubMed] [Google Scholar]
- Hall J. E., Vodyanoy I., Balasubramanian T. M., Marshall G. R. Alamethicin. A rich model for channel behavior. Biophys J. 1984 Jan;45(1):233–247. doi: 10.1016/S0006-3495(84)84151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krouse M. E., Schneider G. T., Gage P. W. A large anion-selective channel has seven conductance levels. Nature. 1986 Jan 2;319(6048):58–60. doi: 10.1038/319058a0. [DOI] [PubMed] [Google Scholar]
- Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- Molle G., Dugast J. Y., Duclohier H., Daumas P., Heitz F., Spach G. Ionophore properties of a synthetic alpha-helical transmembrane fragment of the mitochondrial H+ ATP synthetase of Saccharomyces cerevisiae. Comparison with alamethicin. Biophys J. 1988 Feb;53(2):193–203. doi: 10.1016/S0006-3495(88)83081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G., Stankowski S., Rizzo V. Thermodynamic analysis of incorporation and aggregation in a membrane: application to the pore-forming peptide alamethicin. Biochim Biophys Acta. 1986 Sep 25;861(1):141–151. doi: 10.1016/0005-2736(86)90573-0. [DOI] [PubMed] [Google Scholar]
- Soravia E., Martini G., Zasloff M. Antimicrobial properties of peptides from Xenopus granular gland secretions. FEBS Lett. 1988 Feb 15;228(2):337–340. doi: 10.1016/0014-5793(88)80027-9. [DOI] [PubMed] [Google Scholar]
- Vogel H. Comparison of the conformation and orientation of alamethicin and melittin in lipid membranes. Biochemistry. 1987 Jul 14;26(14):4562–4572. doi: 10.1021/bi00388a060. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]


