Abstract
Type 1 fimbria is a proven virulence factor of uropathogenic Escherichia coli (UPEC), causing urinary tract infections. Expression of the fimbria is regulated at the transcriptional level by a promoter situated on an invertible element, which can exist in one of two different orientations. The orientation of the invertible element that allows the expression of type 1 fimbriae is defined as “on,” and the opposite orientation, in which no transcription occurs, is defined as “off.” During the course of a urinary tract infection, we have observed that the infecting E. coli population alternates between fimbriated and nonfimbriated states, with the fimbriated on orientation peaking at 24 h. We propose that the ability of the invertible element to switch orientations during infection is itself a virulence trait. To test this hypothesis, nucleotide sequence changes were introduced in the left inverted repeat of the invertible element of UPEC pyelonephritis strain CFT073 that locked the invertible elements permanently in either the on or the off orientation. The virulence of these mutants was assessed in the CBA mouse model of ascending urinary tract infection at 4, 24, 48, and 72 h postinoculation (hpi). We conducted independent challenges, in which bladders of mice were inoculated with either a single mutant or the wild type, and cochallenges, in which a mutant and the wild type were inoculated together to allow direct competition in the urinary tract. In both sets of experimental infections, the locked-off mutant was recovered from the urine, bladder, and kidneys in significantly lower numbers than the wild type at 24 hpi (P ≤ 0.05), demonstrating its attenuation. Conversely, the locked-on mutant was recovered in higher numbers than the wild type at 24 hpi (P ≤ 0.05), showing enhanced virulence of this mutant. No significant differences were seen between the mutants and wild type in the urine or the bladder at 48 or 72 hpi. However, the wild type outcompeted the locked-off mutant in the kidneys during the cochallenge experiment at 72 hpi (P = 0.009). Overall, these data suggest that the ability of the invertible element controlling type 1 fimbria expression to phase vary contributes significantly to virulence early (24 hpi) in the course of a urinary tract infection by UPEC and most profoundly influences colonization of the bladder.
Adherence is the first step in bacterial colonization and is necessary for pathogenesis (11). In the colonization of the urinary tract, adherence of Escherichia coli is required to prevent elimination of bacteria by the mechanical action of urine flow. Type 1 fimbriae, expressed on the surface of E. coli and most members of the Enterobacteriaceae (6), bind mannose moieties of uroplakin on the surface of transitional uroepithelial cells lining the bladder (27). Several studies have implicated type 1 fimbriae as a virulence factor integral to the pathogenesis of E. coli in the urinary tract (6, 7, 15, 19, 30). More recently, the expression of type 1 fimbriae has been measured in vivo during the course of an experimental urinary tract infection (UTI) (13, 23, 33).
Expression of type 1 fimbriae is controlled at the level of transcription by an invertible element (1) which contains the promoter responsible for transcription of the structural subunit gene (fimA) and other accessory genes required for the assembly of fimbriae (10, 29). The invertible element, controlled by recombinases FimB and FimE (20), alternates between two orientations. The on orientation places the promoter in a direction that allows for the expression of the fimbriae. In the off orientation, the promoter faces away from the fimbrial genes and prevents transcription. In previous studies, an established PCR assay (36) was modified to quantitatively measure the orientation of the invertible element in an E. coli population in vivo during a UTI (13, 23). This allowed us to determine the percentage of bacteria capable of expressing type 1 fimbriae at defined intervals after the inoculation of the bladder of CBA mice. Other studies in which the orientation of the invertible element of E. coli in the urinary tract was measured have demonstrated dynamic behavior in the switching of the element during the course of infection (13, 23, 33). Additionally, E. coli isolated from patients with cystitis and pyelonephritis was observed to possess different patterns of on and off switching of the invertible element at specific times after transurethral inoculation (13). This finding suggested that the ability to switch the expression of type 1 fimbriae may be necessary for full virulence in the urinary tract. Therefore, the control mechanism for expression, the invertible element, may itself be viewed as a putative virulence factor that dictates the clinical outcome of an infection.
To evaluate the contribution of the invertible element to virulence, we constructed isogenic mutants of uropathogenic E. coli (UPEC) pyelonephritis strain CFT073 that had the invertible element locked either in the on or the off orientation. The locked-off mutant (CFT073OFF) was phenotypically incapable of expressing type 1 fimbriae, while the locked-on mutant (CFT073ON) expressed type 1 fimbriae constitutively. The mutants were tested for virulence in the CBA mouse model of ascending UTI. The locked-on mutant demonstrated enhanced virulence, while the locked-off mutant was attenuated compared to the wild-type strain.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
E. coli CFT073, isolated from the blood and urine of a female patient diagnosed with pyelonephritis, carries the genes for both type 1 and P fimbriae (25). CFT073 can express both fimbrial types as confirmed by mannose-sensitive hemagglutination (MSHA) of guinea pig erythrocytes for type 1 fimbriae and mannose-resistant hemagglutination of human erythrocytes for P fimbriae (13, 26). All bacterial strains used in this study are listed in Table 1. For growth on solid medium, strains were streaked onto Luria agar plates and incubated at 37°C for 18 h. For growth in liquid medium, strains were inoculated into Luria broth and incubated at 37°C for 18 h with aeration (200 rpm).
TABLE 1.
Primers used in this study
| Designation | Sequence (5′→3′) |
|---|---|
| Primer 1 | AGTAATGCTGCTCGTTTTGC |
| Primer 2 | GACAGAGCCGACAGAACAAC |
| 439Nhe | GGGGGCTAGCGTTTGTTACGGGGCAACGGG |
| SK1M | GGGGAGATCTAATTGTCTTGTATTTATTTG |
| LON | GGGGAGATCTATTTTGACTCATAGAGG |
| LOFF | GGGGAGATCTAAACTGTCTATATCATAAATAAG |
| 439Bam | GGGGGATCCAACAACTTCCCCTTTAAAGTG |
| DRA1 | GGGGGATCCGCTGCTTTCCTTTAAAAAAAC |
Recombinant DNA techniques.
Sequencing of plasmid constructs, as well as DNA PCR amplified from the mutant and wild-type strains, was performed by the Biopolymer Lab core facility (University of Maryland, Baltimore) using the dideoxy chain termination method with double-stranded DNA as the template. Reactions were run on a model 373A DNA sequencer (Applied Biosystems, Foster City, Calif.). Plasmid isolation, gel electrophoresis, DNA fragment isolation, ligation, and transformation were carried out according to standard protocols (24).
Construction of the locked-off and locked-on isogenic mutants.
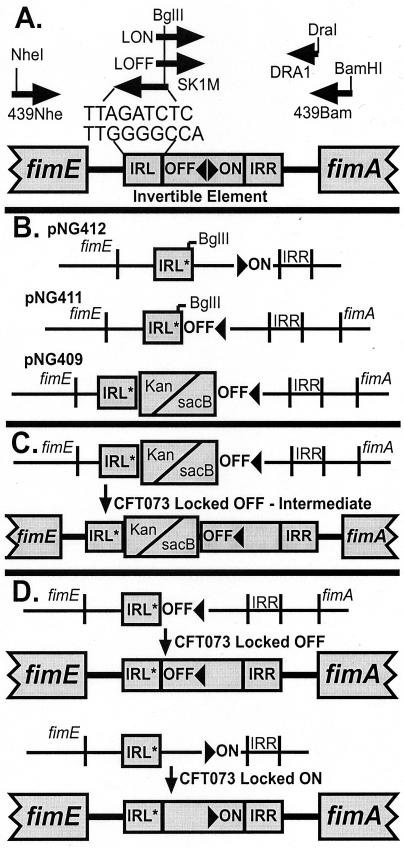
The invertible element region of E. coli CFT073 chromosomal DNA was sequenced upstream through the fimE gene and downstream through the fimA gene. From this DNA sequence, primers 439Nhe and ΔSK1M (Table 1) were synthesized to amplify the CFT073 chromosomal sequence from the left inverted repeat (IRL) of the invertible element through a majority (536 bp) of the fimE gene (Fig. 1A). Likewise, primers 439Bam and LOFF (Table 1) were used to amplify the region of DNA from the IRL through 116 bp of fimA, including the invertible element in the off orientation. Similarly, primers DRA1 and LON (Table 1) amplified the region of DNA beginning at the IRL and terminating just before the first nucleotide of the fimA gene, which included the invertible element in the on orientation. Primers ΔSK1M, LOFF, and LON each introduced a BglII restriction enzyme site into the IRL of the invertible element, effectively mutating 7 of the 9 nucleotides present in the IRL; this resulted in a sequence that did not allow the cloned invertible element to undergo switching.
FIG. 1.
Construction of locked-on and locked-off mutants. (A) PCR amplifications were used both to mutate 7 of the 9 nucleotides in the IRL and to clone the invertible element in either the off or on orientation. Arrows represent primers used for PCR. (B) The locked region of the invertible element downstream of the mutated IRL (IRL∗) and the sequence upstream of the IRL were combined in a series of cloning steps to create on allelic exchange plasmids locked-on (pNG412) and locked-off (pNG411) copies of the invertible element region of the fim operon. Plasmid pNG409 was constructed by inserting the sucrose sensitivity (sacB) and the kanamycin resistance (kan) genes into the BglII site of pNG411. (C) In the first step of a two-step allelic exchange procedure, the wild-type invertible element region of CFT073 was replaced with the locked-off, IRL-mutated invertible element region from plasmid pNG411 that also included the sacB and kan genes used subsequently for selection and counterselection. (D) In the second step of the allelic exchange, the intermediateCFT073 strain had the locked-off invertible element containing kan-sacB genes replaced by a locked-on element from plasmid pNG412 or a locked-off element from the pNG411 version of the invertible element, containing only the mutations in the IRL. See Materials and Methods for further details. IRR, inverted repeat right.
The PCR products from each of the three sets of promoters were directly cloned into PCR-Blunt (Stratagene). The cloned product of PCR amplification by primers 439Nhe and ΔSK1M was designated as pNG400; the cloned product of 439Bam and LOFF was designated as pNG401; and the cloned product of DRA1 and LON was designated as pNG402 (Table 2). Plasmid pNG400 was digested with restriction enzymes BglII and PstI, and the resulting fragment (∼594 bp) was gel purified away from the rest of the plasmid and ligated into pNG401 and pNG402, which had been previously digested with BglII and PstI. The resulting construct, pNG401, now also containing the BglII/PstI fragment from pNG400, was designated as pNG403, and pNG402, now containing the BglII/PstI fragment, was designated as pNG404. Plasmid pNG403 was digested with restriction enzymes BamHI and NheI, and the 1,124-bp fragment from the digest was gel purified away from the rest of the plasmid. The 1,124-bp fragment was subsequently cloned into pHK184 (I. Blomfield, unpublished cloning vector) previously digested with BamHI and NheI. The new plasmid constructed from the fragment cloned from pNG403 into pHK184 was designated as pNG405. Plasmid pIB279 (5) was digested with BamHI and a 3.8-kb fragment containing the kanamycin gene (kan), and the sucrose sensitivity sacB gene from B. subtilis was gel purified away from the rest of the plasmid. The resulting fragment was cloned into the previously engineered BglII site of the ΔIRL in pNG405; the resulting plasmid was designated as pNG407. Plasmid pNG407 has two origins of replication, a pUC origin and a temperature-sensitive pSC101 origin which functions only at temperatures below 32°C. Plasmid pNG407 was digested with the restriction enzyme SacI to remove the pUC origin of replication. The SacI fragment was gel purified away, and the plasmid was religated to form a temperature-sensitive version of pNG407, which was designated as pNG409 (Fig. 1B). The pUC origin of replication was also removed from pNG405 to create the temperature-sensitive plasmid pNG411 (Fig. 1B). The 1,020-bp fragment from pNG404 with the invertible element locked in the on orientation was cloned directly into a version of pHK184 that already had the pUC origin removed, leaving only the temperature-sensitive origin; this plasmid was designated as pNG412 (Fig. 1B). The 3.8-kb kan-sacB fragment from pIB279 was cloned into the BglII site of the ΔIRL in pNG412, thus creating pNG410.
TABLE 2.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype (comments) | Source or reference |
|---|---|---|
| E. coli strains | ||
| CFT073 | Pyelonephritis isolate, fim+pap+hly+ | 13 |
| CFT073OFF-intermediate | Δ IRL, fim invertible element locked off, Kanr, sacB gene | This work |
| CFT073OFF | Δ IRL, fim invertible element locked off | This work |
| CFT073ON | Δ IRL, fim invertible element locked on | This work |
| CFT073nal | Nalidixic acid-resistant derivative of wild-type CFT073 (equally virulent as wild-type strain in CBA mouse model of ascending UTI) | 26 |
| AAEC 189 | ΔfimB-H ΔrecA endA | 4 |
| Top10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) Φ80lacZΔM15 ΔlacX74 recA1 deoR araD139 Δ(ara-leu)7697 galU galK rspL (Strr) endA1 nupG | Invitrogen |
| Plasmids | ||
| pNG400 | 594-bp fragment from primers 439Nhe and ΔSK1M, ΔIRL, in PCR-BluntII-Topo | This work |
| pNG401 | 530-bp fragment from primers 439Bam and LOFF, ΔIRL, in PCR-BluntII-Topo | This work |
| pNG402 | 426-bp fragment from primers DRA1 and LON, ΔIRL, in PCR-BluntII-Topo | This work |
| pNG403 | 594-bp (439Nhe/ΔSK1M) fragment from pNG400 cloned into the BglII-PstI sites of pNG401, ΔIRL | This work |
| pNG404 | 594-bp (439Nhe/ΔSK1M) fragment from pNG400 cloned into the BglII-PstI sites of pNG402, ΔIRL | This work |
| pNG405 | 1,124-bp fragment from pNG403 cloned into the NheI-BamHI sites of pHK184, ΔIRL (locked off) | This work |
| pNG407 | kan-sacB fragment from pIB279 cloned into the BglII site of pNG405, ΔIRL (locked off) | This work |
| pNG409 | pNG407 with a ΔpUC origin of replication, temperature-sensitive pSC101 origin | This work |
| pNG410 | kan-sacB fragment from pIB279 cloned into the BglII site of pNG412, ΔIRL (locked off) | This work |
| pNG411 | pNG405 with a ΔpUC origin of replication, temperature-sensitive pSC101 origin | This work |
| pNG412 | 1,020-bp fragment from pNG404 cloned into the NheI-BamHI sites of temperature sensitive vector pHK184(ΔpUC origin), ΔIRL (locked on) | This work |
| pHK184 | Camr, temperature-sensitive pSC101 origin of replication, pUC origin of replication | Ian Blomfield, un- published data |
| pIB279 | Ampr, Kanr, sacB-Neor cassette | 5 |
| PCR-BluntII-Topo | pMB1 origin of replication, kanamycin- and zeocin-resistant, ccdB lethal gene for selection of inserts | Invitrogen |
Plasmids pNG409, pNG410, pNG411, and pNG412 were subsequently used in a two-step selection-counterselection allelic exchange procedure as previously described (4). In the first allelic exchange, pNG409 and pNG410 were used to replace the wild-type invertible element of the pyelonephritis strain CFT073 with a locked-off or locked-on version of the invertible element, both of which were interrupted with the kan-sacB genes. Growth on Luria agar containing kanamycin (50 μg/ml) was used to select for successful allelic exchange. Only pNG409 produced a successful allelic exchange, resulting in the mutated strain designated CFT073OFF-intermediate (Fig. 1C). In the second allelic exchange, pNG411 and pNG412 were used to replace the locked-off invertible element (containing kan-sacB) of CFT073OFF-intermediate with a locked-off or locked-on invertible element that did not have the kan-sacB genes and differed from the wild-type strain of CFT073 by only 7 nucleotides. Counterselection using sucrose sensitivity conferred by the sacB gene allowed for selection of successful allelic exchange. Both pNG411 and pNG412 resulted in successful allelic exchanges, which produced the isogenic mutants CFT073OFF and CFT073ON (Fig. 1D).
The fim invertible element region of the resulting mutant strains and the plasmid constructs were PCR amplified and sequenced to confirm the presence of the desired mutations and the absence of any undesired secondary mutations. Plasmids pNG400, -401, -402, -403, and -404 were maintained in E. coli strain Top10 (Invitrogen, Carlsbad, Calif.). Plasmids pNG405, -407, -409, -410, -411, and -412 were maintained in E. coli strain AAEC189.
Invertible element PCR assay.
Pyelonephritis isolate CFT073 and the isogenic mutants CFT073OFF and CFT073ON were incubated statically in Luria broth at 37°C, 18 h. Urine samples from mice that had been transurethrally inoculated individually with each of the strains were collected at 72 h postinfection. The invertible-element PCR assay was conducted on these samples as described previously (23). In brief, primer 1 and primer 2 (Table 2) were used to amplify the 601-bp invertible element. Digestion of the PCR products with SnaBI and electrophoresis on a 2% agarose-1× TAE (Tris-acetate-EDTA buffer) gel allowed determination of the orientation of the invertible element. Bands of specific size (see Results) indicated the proportion of the invertible elements that were in the on or off orientation.
MSHA assay.
CFT073, CFT073OFF, and CFT073ON were cultured both in Luria broth statically for 18 h at 37°C and on Luria agar for 18 h at 37°C. Bacteria resuspended from the plate and from broth were adjusted in phosphate-buffered saline (PBS) to a concentration of 109 CFU/ml and assayed for expression of type 1 fimbriae by hemagglutination. Bacterial suspensions were serially diluted twofold into round-bottom 96-well microtiter plates in duplicate. Equal volumes of 3% (vol/vol) guinea pig erythrocytes were mixed with bacterial suspensions. Mannose was added to a 50 mM final concentration to one row of the duplicated serial dilutions for each bacterial growth condition. Nonagglutinated guinea pig erythrocytes formed tight buttons of cells at the bottom of the plate well, whereas agglutinated erythrocytes formed a diffuse mat of cells across the bottom of the well. The titer was defined as the reciprocal of the highest dilution of bacteria that agglutinated erythrocytes.
Murine model of ascending UTI.
Six- to eight-week-old female CBA mice (20 to 22 g; Harlan Sprague Dawley, Indianapolis, Ind.) were anesthetized with pentobarbital sodium (Nembutal) and inoculated transurethrally with a 50-μl bacterial suspension containing approximately 107 CFU of E. coli using a 0.28-mm-diameter sterile polyethylene catheter connected to an infusion pump (Harvard Apparatus, Millis, Mass.) as described previously (23). For individual challenges, inocula were prepared from bacteria grown on Luria agar plates incubated at 37°C for 18 h. The resulting bacterial lawns from three plates were suspended in 2 ml of PBS, adjusted so that a 50-μl volume contained the desired inoculum. Mice were sacrificed at 4, 24, 48, and 72 h postinoculation (hpi), and the bladder and kidneys were aseptically removed, weighed, homogenized (sterile glass grinder [Kontes, Inc., Vineland, N.J.]), and diluted threefold in PBS. Numbers of bacteria present in the samples were then determined by growth on Trypticase soy agar after spiral plating (Spiral Systems, Bethesda, Md.).
Cochallenge in the murine model of ascending UTI.
Mice were inoculated transurethrally as described above, delivering 50 μl, containing approximately 109 CFU E. coli, to the bladder. The inocula were prepared by growing lawns of E. coli strain CFT073nal on five Luria agar slants containing nalidixic acid (50 μg/ml) and incubated for 18 h at 37°C. Lawns of CFT073ON and CFT073OFF mutants were similarly grown on Luria agar slants without antibiotics. These bacterial lawns for each strain were suspended in 4 ml of PBS. For the competition experiment between the wild type and the locked-on mutant, suspensions of CFT073nal and CFT073ON were mixed in approximately a 1:1 ratio for the inoculum. The same was done for CFT073nal and CFT073OFF for the wild type versus locked-off competition. At 4, 24, 48, and 72 hpi, urine samples were collected from eight mice before sacrifice by lethal dose of methoxyflurane. Bladder and kidneys were aseptically removed and homogenized. Samples were cultured on both plain Luria agar plates and those containing nalidixic acid (50 μg/ml) to determine the number of CFU per milliliter of urine or per gram of tissue for each strain.
RESULTS
Genotypes and phenotypes of E. coli CFT073, CFT073OFF, and CFT073ON.
Locked-on (CFT073ON) and locked-off (CFT073OFF) mutants were constructed as described in Materials and Methods by altering the sequence of the IRL within the invertible element carrying the type 1 fimbrial promoter (Fig. 1). Sequencing of the invertible element and flanking regions of the locked-on and locked-off isogenic mutants allowed us to confirm the site of the desired mutation, where we changed the wild-type sequence of 5′-TTGGGGCCA-3′ to 5′-TTAGATCTC-3′. No undesired secondary mutations were detected by sequencing of PCR-amplified chromosomal DNA of the mutants over the area of the allelic exchange. Specific pitfalls in the construction of the mutant were encountered but overcome; these difficulties are expanded upon in the Discussion.
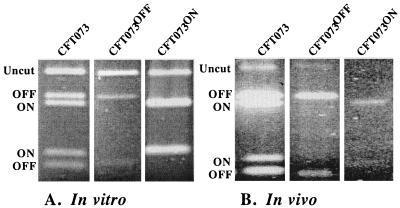
The genotype of the mutants was confirmed by means of the established PCR assay (23) after both in vitro and in vivo passage to demonstrate that reversion to wild type had not occurred. In brief, primer 1 and primer 2 (Table 1) were used to PCR amplify a 601-bp fragment carrying the fim invertible element. Asymmetric digestion with SnaBI allows the visualization of the invertible element in the off position, yielding fragments of 403 and 198 bp; the on position yields fragments of 440 and 161 bp. The invertible element of the mutants was found locked in place and not capable of switching after passage of the mutants in Luria broth, statically for 18 h (Fig. 2A). Likewise, CFT073, CFT073ON, and CFT073OFF were transurethrally inoculated into mice; after 72 h, urine was recovered and subjected to PCR analysis. As expected, CFT073 revealed the invertible element orientation as a mixture of on and off; however, CFT073ON remained switched on and CFT073OFF remained switched off (Fig. 2B). CFT073OFF was incapable of MSHA of guinea pig erythrocytes (Table 3), the phenotypic assay for type 1 fimbria expression (9). CFT073ON demonstrated strong MSHA under all culture conditions (Table 3). The growth rates of CFT073 and the phase-locked mutants, cultured independently in Luria broth at 37°C with aeration, were not significantly different (data not shown). CFT073 and each isogenic mutant grew equally as well when mixed together in broth. Starting with a mutant/wild-type ratio equal to 1, after daily passage in Luria broth in vitro for 72 h, the locked-off/wild-type ratio equaled 1.22; the locked-on/wild-type ratio was 1.15. Thus, neither mutant was outcompeted after 72 h of coculture.
FIG. 2.
Genotypic characterization of invertible element orientation in phase-locked mutants. E. coli CFT073 and its isogenic phase-locked mutants were cultured for 18 h statically in Luria broth and used directly for PCR (A) or transurethrally inoculated independently into mice, whose urine collected at 72 hpi was used for PCR (B). The invertible element amplified by PCR was digested with SnaBI; following both in vitro and in vivo growth, asymmetric cutting demonstrates that the orientation of the invertible element in the parent strain CFT073 is both in the on and off position. CFT073OFF remains only in the off orientation; CFT073ON remains only in the on orientation.
TABLE 3.
Hemagglutination of guinea pig erythrocytes by E. coli CFT073 and phase-locked mutants
| Strain | Growth in broth
|
Growth in agar
|
||
|---|---|---|---|---|
| Titera | Hemagglutination in presence of 50 mM mannose | Titer | Hemagglutination in presence of 50 mM mannose | |
| CFT073 | 64 | —b | — | — |
| CFT073ON | 128 | — | 64 | — |
| CFT073OFF | — | — | — | — |
Titers are the reciprocal of the highest dilution of bacteria that gave agglutination, starting with 109 CFU.
—, no agglutination.
Colonization of the locked-off mutant in the murine model of ascending UTI.
A suspension of 107 CFU of the locked-off mutant of parent pyelonephritis strain CFT073 was transurethrally inoculated into the bladders of CBA mice (15). Mice were sacrificed at 4, 24, 48, and 72 hpi, and bladders and kidneys were removed and used to determine the number of CFU per gram of tissue for the mutant strain. Urine samples were also collected from the mice just prior to sacrifice and used to quantify CFU. Results were compared to those for urine, bladder, and kidney samples from mice infected at the same time and in the same fashion with the wild-type strain CFT073 (Fig. 3A to C).
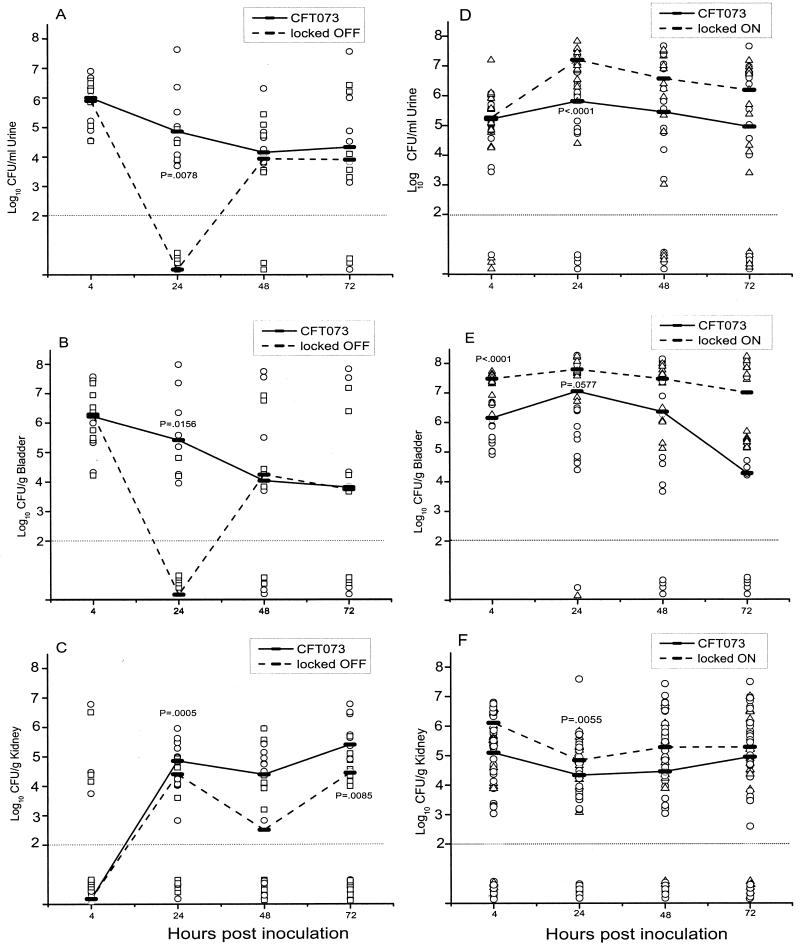
FIG. 3.
Independent challenges with mutants and wild type in UTI. Mice were transurethrally inoculated independently with 107 CFU of E. coli CFT073 wild type (○), CFT073ON (▵), or CFT073OFF (□). Urine (A and D), bladder (B and E), and kidney (C and F) samples were quantitatively cultured at 4, 24, 48, and 72 hpi. Each symbol represents one sample from each mouse. The horizontal dotted line at 102 CFU indicates the lower limit of detection. Median values for each time point are connected by a solid line (CFT073) or dashed line (mutant).
CFT073OFF tended to be recovered in lower numbers (>3 logs) in the urine of the mice than those infected with the parent strain CFT073 at 24 hpi (Fig. 3A) (P = 0.056). Samples collected at 4, 48, and 72 hpi did not reveal significant differences between the numbers of locked-off and parent strains. The lack of a clear significant difference between the median values at 24 hpi is likely due to the low number of urine samples (n = 5) available at each time point.
Bacterial counts of CFT073OFF in the bladder were significantly lower than those of wild-type CFT073 at 24 and 48 hpi (Fig. 3B), with a >3-log difference in the median bacterial counts between the locked-off mutant and wild-type E. coli strains at 24 hpi (P = 0.003). At 48 hpi, the mutant numbers were nearly 2 logs lower than wild-type numbers (P = 0.02). The median numbers of bacteria in the bladders did not differ significantly between the two strains at 4 and 72 hpi.
Samples taken at 24 h p.i from kidneys yielded a significantly (3-log) greater median number of wild-type strain CFT073 than the mutant CFT073OFF (Fig. 3C) (P = 0.032). The median bacterial numbers of the two strains at 4, 48, and 72 hpi were only marginally different and not significant. For each of the three sampling sites (urine, bladder, and kidney), the significant differences between the mutant and wild-type strains occurred at the earlier time-points of infection, most notably at 24 hpi (Fig. 3A to C).
Colonization of the locked-on mutant in the murine model of ascending UTI.
The ability of the locked-on mutant to colonize the urinary tract was compared to that of the wild-type strain of CFT073 in a series of experimental murine infections identical to the experiments performed with the locked-off mutant (Fig. 3D to F).
Twenty-four hours after inoculation, the median bacterial number of CFT073ON present in the urine was nearly 2 logs greater than the median number of wild-type CFT073 in similar samples (P = 0.02) (Fig. 3D). However, this difference between the two strains rapidly disappeared by 48 hpi. Urine samples showed no significant differences between the two strains at 4 and 72 hpi.
A comparison of the median bacterial numbers of CFT073ON and the parental strain CFT073 in the bladders of mice demonstrated an advantage in colonization for the locked-on mutant early in colonization. (Fig. 3E). Similar to the numbers in the urine, the median bacterial number for CFT073ON was nearly 2 logs greater than the median value of the parental strain at 24 hpi (P < 0.05). This advantage of the locked-on mutant dissipated at 48 hpi when the median bacterial numbers of the locked-on mutant did not differ significantly from those of the wild-type strain. The differences observed between the parent and locked-on strain at 4 and 72 hpi were also not significant.
Bacterial colonization of the kidneys in mice differs from what was observed in the urine and bladder samples collected from the same animals (Fig. 3F). The median numbers of wild-type CFT073 and locked-on mutants colonizing the kidneys of mice were not significantly different at any time point during the course of the experimental UTIs. However, at 4 hpi, the median bacterial number of wild-type strain CFT073 tended to be higher (2 logs greater) than that of CFT073ON (P = 0.069).
Coinfection with CFT073nal and CFT073OFF.
A more-sensitive method to assay for the effects of type 1 fimbriae on virulence is cochallenge, rather than individual challenge, of mice with wild-type and mutant strains (22). To this end, the bladders of CBA mice were transurethrally inoculated with a total of 109 CFU of CFT073nal (nalidixic acid-resistant derivative of wild type) and CFT073OFF; strains were mixed in a 1:1 ratio. At 4, 24, 48, and 72 hpi, urine samples were collected and mice were sacrificed to obtain the bladder and kidneys. The number of CFU per milliliter of urine or per gram of tissue was determined at each time point. Median values were identified, and the Wilcoxon matched-pairs test of nonparametric data was used to compare the viable counts for CFT073nal and CFT073OFF within each sample (Fig. 4A to C).
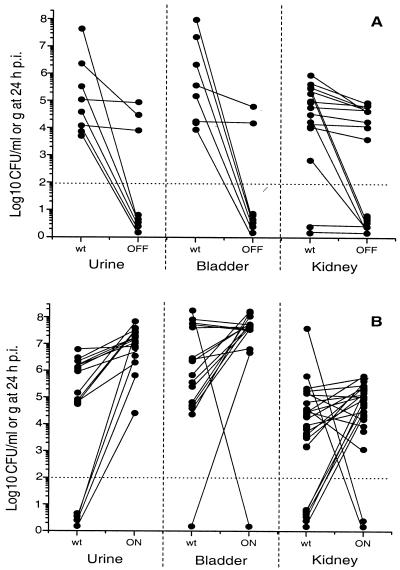
FIG. 4.
Cochallenges with mutants and wild type in the murine model of ascending UTI. Mice were transurethrally cochallenged with a total inoculum of 109 CFU containing a 1:1 ratio of E. coli CFT073 wild type (○) and CFT073ON (▵) or E. coli CFT073 wild type (○) and CFT073OFF (□). Urine (A and D), bladder (B and E), and kidney (C and F) samples were quantitatively cultured at 4, 24, 48, and 72 hpi. Each sample from individual mice yielded a value for the wild-type strain and its isogenic phase-locked mutant. The horizontal dotted line at 102 CFU indicates the lower limit of detection. Median values for each time point are connected by a solid line (CFT073) or dashed line (mutant).
In the urine, the median bacterial counts of the locked-off mutant were 3 logs lower than the those of the wild type at 24 hpi (P = 0.0078) (Fig. 5A). However, there were no significant differences at 4, 48, or 72 hpi (Fig. 4A).
FIG. 5.
Competition of wild type and phase-locked type 1 fimbrial mutants of E. coli CFT073 in the mouse model of ascending UTI at 24 hpi. Mice were transurethrally cochallenged with wild-type CFT073 (wt) and its isogenic locked off mutant (OFF) (A) or wild-type CFT073 and the locked on mutant (ON) (B). Urine, bladder, and kidney samples were quantitatively cultured. Data are shown for samples taken 24 hpi; a line connects values from the same mouse. The horizontal dotted line at 102 CFU indicates the lower limit of detection.
Reflecting the situation in the urine, CFT073OFF was recovered in significantly lower numbers than the wild-type CFT073nal strain at 24 hpi in the bladder (P = 0.016) (Fig. 5A). The difference between the medians was greater than 3 logs. There were no significant differences between medians for the mutant and the wild type at 4, 48, or 72 hpi (Fig. 4B).
The median bacterial counts in the kidneys for both CFT073nal and CFT073OFF were below the level of detection (<100 CFU) at 4 hpi. By 24 hpi, counts had risen to detectable levels, and a statistical significance was shown at 24 hpi when the wild-type strain predominated in numbers higher than its isogenic mutant, where the expression of type 1 fimbriae is locked off (P = 0.0005) (Fig. 5A). CFT073nal continued to predominate, with a 2-log difference between the medians at 48 hpi, and significance was again shown at 72 hpi over CFT073OFF (P = 0.0085). Thus, in the urine, bladder, and kidney, the wild-type strain CFT073nal clearly outcompeted CFT073OFF 24 h after inoculation (Fig. 4C).
Coinfection of CFT073nal and CFT073ON.
CBA mice were inoculated with a suspension of CFT073nal and CFT073ON, mixed in a 1:1 ratio; the total inoculum was 109 CFU. These cochallenge experiments were carried out in a manner identical to the cochallenge with CFT073OFF described above. The numbers of CFU per milliliter of urine and per gram of tissue were determined at 4, 24, 48, and 72 hpi (Fig. 4D to F).
In urine, the locked-on strain was recovered in higher numbers than the wild type at 24 hpi (P < 0.0001) (Fig. 5B). No significant differences were observed at 48 and 72 hpi. Additionally, there was no statistical difference between mutant and parent strains in the urine at 4 hpi (Fig. 4D).
In the bladder, the likely constitutive expression of type 1 fimbriae led to the predominance in bacterial counts of CFT073ON over the wild-type CFT073nal at the earliest time point of 4 hpi, which is unlike the results observed in the urine at this time. The median counts showed a >1-log difference between the two strains (P < 0.0001). By 24 hpi, the counts for the locked-on mutant tended to be higher than those for the wild type (P = 0.058) (Fig. 5B), and the general trend continued that the locked-on strain demonstrated an advantage in colonization of the bladder over the wild type. There were no significant differences at 48 or 72 hpi (Fig. 4E).
The median bacterial counts in the kidneys again showed a higher level of colonization for the locked-on strain when compared to CFT073nal at 24 hpi (P = 0.0055) (Fig. 5B). There was no difference between the mutant and the wild-type strains at 4, 48, or 72 hpi (Fig. 4F).
DISCUSSION
Type 1 fimbria is a well established virulence factor of UPEC, for which the molecular Koch's postulates of microbial pathogenesis have been satisfied (7). Previously in our laboratory, it was found that the pattern of switching of the invertible element correlates with the type of UTI from which the strain was isolated. That is, during experimental infections, cystitis strains switched the invertible element to the on position, where it remained; pyelonephritis strains turned the switch on (to a lesser degree) early in infection and then tended to turn the switch off (13). Therefore, we hypothesized that switching of the invertible element itself contributes significantly to virulence in the urinary tract. To test this, mutants in which the invertible element is locked either in the on or off position were constructed in UPEC strain CFT073, originally isolated from the urine and blood of a female patient with pyelonephritis (25). Experimental infections in the murine model using these mutants demonstrate that type 1 fimbriae displayed on the surface make the bacterium more fit for colonization of the urinary tract. The locked-on mutant is more virulent than the wild-type strain, and the wild type is subsequently more virulent than the locked-off mutant. The strain that expresses more type 1 fimbriae is found in higher median numbers in the urinary tract than the strain expressing less (or none) of the adhesin, as shown at 24 hpi. These data suggest that (i) type 1 fimbriae are most critical for virulence at early times during infection and (ii) the ability to phase vary at specific times postinfection gives the invertible element itself an important role in determining virulence.
The mutants used in this study were constructed with specific mutations in the IRL of the invertible element that locked the element in either the on (CFT073ON) or off (CFT073OFF) orientation. In genotypic and hemagglutination assays, neither mutant was found capable of switching the orientation of their invertible element. It is notable that the construction of the locked-on mutant strain is fraught with obstacles. When the locked-on invertible element is originally cloned onto a plasmid, it is important that no part of the fimA gene be cloned onto the plasmid behind the invertible element. The presence of the fimA genetic material (as little as 100 bp) results in the recovery of clones with mutations in the first nucleotide position of the −35 region of the promoter and, in most cases, a second mutation in the noncoding region between the inverted repeat right and the start of the fimA gene. These mutations result in a loss of type 1 fimbria expression in the final mutant. For this reason, a smaller fragment was cloned using primers that did not localize within fimA (Fig. 1). Additionally, construction of an intermediate locked-on strain containing the counterselectable sacB gene and the gene for kanamycin resistance proved to be unachievable. Other laboratories have also attempted to construct a locked-on strain by this method and have noted difficulties recovering a locked-on intermediate strain, thus blocking construction of phase-locked mutants (Ian Blomfield, personal communication). It was still possible to construct the final locked-on mutant using the locked-off strain, which was easier to construct, as the intermediate replacement strain (Table 2). This was made possible by the excellent screen afforded by the counter-selection against the sacB gene. A reason why the locked-on strain intermediate could not be constructed is not immediately apparent.
Both locked-off and locked-on mutant strains demonstrated significant differences from the parent strain CFT073 during the course of a UTI in individual challenges. The locked-on mutant colonized the urine and bladder of mice with median numbers ∼2 logs greater than that of the wild-type strain at 24 hpi. Also at 24 hpi, the locked-off mutant colonized the bladders, urine, and kidneys of mice, on average, in numbers 2 to 3 logs lower than did the wild-type strain. Later in the course of the infection, at 48 and 72 hpi, the differences in median bacterial numbers between CFT073 and each mutant, respectively, decreased until there was no statistically significant difference between the two isogenic strains (Fig. 3).
These mutants were tested in cochallenge studies to further investigate the relative contribution of the orientation of the invertible element to virulence. The wild type and each respective mutant were inoculated into the bladder together and allowed to directly compete, therefore giving this assessment of virulence more sensitivity than possible in individual challenges. The results for the locked-on strain and the wild type and the results for the and locked-off strain and the wild type share similarities. In the bladder, urine, and kidneys for both sets of data, the strains expressing more type 1 fimbriae predominate in numbers (P ≤ 0.05) and at 24 hpi. Generally, at other times postinfection (4, 48, and 72 hpi), no statistical significance between strains was observed (Fig. 4).
Since type 1 fimbriae are well established as a virulence factor in UPEC strains colonizing the urinary tract, it is not surprising that the locked-off mutant strain would colonize the mouse model of UTI in significantly lower numbers than the wild-type. CFT073OFF is similar in nature to other type 1 fimbria-null mutants (7, 17, 18). In the previous studies at a time period from 3 to 48 hpi, the type 1 null mutants exhibited up to a 3-log decrease in colonization of the bladder compared to wild-type strains. A 2-log decrease in the colonization of the kidneys was also reported in the previous studies. These data are similar to the results reported here for the locked-off mutant. While the previous studies stopped at 48 hpi, this study monitored the mutant until 72 h after transurethral inoculation and demonstrated that there are no significant differences between the mutant and the wild-type strains at this later stage. This new observation indicates that type 1 fimbriae are important to colonization during the early stages of the infection (24 hpi), and the continued expression of type 1 fimbriae at later stages provides little advantage to virulence. This proposition is in agreement with other reports that have demonstrated a role for type 1 fimbria binding to the uroepithelial cells of the bladder during the early stages of a UTI (13, 27).
This begs the question of why a virulence factor so advantageous early in infection can become unnecessary, at least for this highly virulent strain, later in the same infection. The persistent expression of type 1 fimbriae, which serve as excellent antigens (21), might elicit a strong response from the host immune system that would effectively eliminate the bacteria. Clearly an immune response is directed against type 1 fimbriae during a UTI (2, 3, 12, 16, 32, 34). However the time necessary for a host to mount a strong immune response against type 1 fimbriae may be longer than 72 hpi, so this explanation may not apply to our observations.
Subsequent expression of other fimbrial types provides another possible explanation for why type 1 fimbria expression is important only at the early stage of a UTI. When type 1 fimbriae are expressed, as many as 500 fimbriae cover the surface of the bacteria, accounting for ∼8% of the total protein of the cell (31). Reduced space and resources to express other types of fimbriae are left. An earlier study suggested that the expression of different fimbriae on the surface of E. coli occurs, with only one fimbrial type being expressed at any given time (28). A more recent study has demonstrated cross talk between the regulation of the type 1 fimbria operon and the P-fimbria operon (35). PapB, a regulatory gene product of the P-fimbria operon, down-regulates the expression of the genes necessary for type 1 fimbriae. P fimbriae are believed to contribute to the colonization of the kidneys, and therefore E. coli virulence, in pyelonephritis (8, 14). Therefore, it would be logical for E. coli to express type 1 fimbriae to colonize the bladder early in UTI, but as the bacteria ascend the ureter, they may need to switch to the expression of P fimbriae for successful kidney colonization. In the case of the locked-on mutant, continued expression of type 1 fimbriae may therefore interfere with P-fimbria expression. This may account for the trend in the delay of CFT073ON colonizing the kidney compared to CFT073 (Fig. 3F). The creation of locked-on and -off mutants in CFT073 also containing a null mutation of P-fimbriae may elucidate the coordinated expression of the two fimbriae.
The method of preparation of the bacterial inoculum for transurethral inoculation should be considered when evaluating the outcomes of these experimental infections. The strains were grown on solid medium, which does not allow for expression of type 1 fimbriae in the wild-type strain (≥98% off) (13). Thus, the wild type and locked-off mutant are both introduced into the bladder of the mouse with the invertible element in the off position. However, the locked-on strain always expresses type 1 fimbriae, even on solid medium, which may give an initial advantage to these bacteria. This may explain our results from the cochallenge experiment of locked-on mutant and wild type, where at 4 hpi the locked-on mutant is recovered in higher median numbers in the bladder (P < 0.0001). In the cochallenge between locked-off mutant and wild type, there was no significant difference at 4 hpi, since both bacteria were introduced into the bladder with their invertible element in the off orientation.
In a previous study, wild-type strain CFT073 was introduced into the mouse urinary tract with ≥98% of the invertible elements in the off orientation. By 24 hpi, a median value of 33% of the CFT073 population had switched their invertible elements to the on orientation, allowing for expression of type 1 fimbriae. At 48, 72, and 96 hpi, ≤2% of CFT073 had the invertible element in the on orientation (13). In light of these data, our results are even more striking. We know that the locked-on mutant has its invertible element in the on position in 100% of the population at all times. The wild-type strain has only a third of its population in the on position at 24 hpi, and yet the most-significant difference still occurs at this time point, as opposed to 4, 48, and 72 hpi when the two strains have nearly opposite orientations of their invertible elements. In the challenges using the locked-off mutant, the only difference in median counts between the two strains comes at 24 hpi, again when the wild type has 33% of its invertible elements in the on position. At all other time points the medians for the wild type and locked-off mutant are not significantly different, due to the lack of type 1 fimbria expression in both.
When comparing our results within this study, it becomes apparent that there is a strong correlation between the results in the bladder and the urine. The trends seen in the bladder can predict those in the urine, and vice versa. This coincides with a previous study that found that the percentage of bacteria in the urine with the invertible elements in the on orientation correlates with the number of CFU found per gram of bladder (r = 0.59) (13). There seems to be no correlation between the numbers of bacteria in the kidney with those in either the bladder or the urine.
In the individual challenges, there was statistical significance in median numbers at 24 hpi for the wild type over those for the locked-off mutant, but not for the locked-on mutant over the wild type. However, in the cochallenges for both the locked-on and locked-off mutants, there was statistical significance for expression of type 1 fimbriae at 24 hpi (Fig. 5). This cochallenge experiment showed more sensitivity than the individual challenges due to the direct competition of the mutant and the wild-type strain, and thus the advantage of expressing type 1 fimbriae early in the kidney is revealed. In late kidney infection, the results are not as readily explainable. It is solely in the cochallenge experiment, where the wild type was recovered in median numbers 2 log higher than those for the locked-off mutant at 48 hpi, and then statistical significance was reached at 72 hpi. This apparent importance of type 1 fimbriae in late kidney infection might be accounted for by the necessity of colonizing the bladder prior to kidney colonization. A scenario is proposed in which bacteria with their invertible elements locked on in the bladder are selected for and thus are the predominant strain available for ascent into the kidneys. It may therefore be informative to perform an experiment in which an inoculum of wild type mixed with either the locked-on or -off mutant is injected directly into the kidney. This would eliminate any influence of the reservoir of bacteria in the bladder, to determine more definitively which strain would prevail in the kidneys. However, an advantage conveyed upon bacteria expressing type 1 fimbriae in late infection in the kidney cannot be ruled out at this time.
It is important to note that the locked-on and -off mutants have been constructed in only one strain, CFT073, a highly virulent pyelonephritis isolate. It would be informative to construct similar locked-on and -off mutants in a cystitis isolate, such as strain F11 (13). This cystitis isolate has previously demonstrated a pattern of switching of the invertible element in vivo distinctly different from that observed for the pyelonephritis strain CFT073 (13). Since F11 invertible elements remain mostly in the on orientation (61 to 98% on) from 24 through 96 hpi, perhaps the expression of type 1 fimbriae here may continue to play an important role in virulence into late UTI infection. Such research may clarify the role of the invertible element of type 1 fimbriae in contributing to the clinical manifestations of a UTI.
Acknowledgments
This work was supported in part by Public Health Service grant AI43363 from the National Institutes of Health.
Editor: A. D. O'Brien
REFERENCES
- 1.Abraham, J. M., C. S. Freitag, J. R. Clements, and B. I. Eisenstein. 1985. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc. Natl. Acad. Sci. USA 82:5724-5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham, S., J. Shin, and R. Malaviya. 2001. Type 1 fimbriated Escherichia coli-mast cell interactions in cystitis. J. Infect. Dis. 183(Suppl. 1):S51-S55. [DOI] [PubMed] [Google Scholar]
- 3.Agace, W. W., M. Patarroyo, M. Svensson, E. Carlemalm, and C. Svanborg. 1995. Escherichia coli induces transuroepithelial neutrophil migration by an intercellular adhesion molecule-1-dependent mechanism. Infect. Immun. 63:4054-4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomfield, I. C., M. S. McClain, and B. I. Eisenstein. 1991. Type 1 fimbriae mutants of Escherichia coli K12: characterization of recognized afimbriate strains and construction of new fim deletion mutants. Mol. Microbiol. 5:1439-1445. [DOI] [PubMed] [Google Scholar]
- 5.Blomfield, I. C., V. Vaughn, R. F. Rest, and B. I. Eisenstein. 1991. Allelic exchange in Escherichia coli using the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol. Microbiol. 5:1447-1457. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan, K., S. Falkow, R. A. Hull, and S. I. Hull. 1985. Frequency among Enterobacteriaceae of the DNA sequences encoding type 1 pili. J. Bacteriol. 162:799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connell, I., W. Agace, P. Klemm, M. Schembri, S. Marild, and C. Svanborg. 1996. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. USA 93:9827-9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnenberg, M. S., and R. A. Welch. 1996. Virulence determinants of uropathogenic Escherichia coli, p. 135-174. In H. L. T. Mobley and J. W. Warren (ed.), Urinary tract infections: molecular pathogenesis and clinical management. ASM Press, Washington, D.C.
- 9.Duguid, J. P. O. 1980. Adhesive properties of Enterobacteriaceae, p. 186-217. In E. H. Beachey (ed.), Bacterial adherence, receptors, and recognition, series B 6. Chapman and Hall, Ltd., London, United Kingdom.
- 10.Eisenstein, B. I. 1981. Phase variation of type1. fimbriae in Escherichia coli is under transcriptional control. Science 214:337-339. [DOI] [PubMed] [Google Scholar]
- 11.Finlay, B. B., and S. Falkow. 1989. Common themes in microbial pathogenicity. Microbiol. Rev. 53:210-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godaly, G., B. Frendeus, A. Proudfoot, M. Svensson, P. Klemm, and C. Svanborg. 1998. Role of fimbriae-mediated adherence for neutrophil migration across Escherichia coli-infected epithelial cell layers. Mol. Microbiol. 30:725-735. [DOI] [PubMed] [Google Scholar]
- 13.Gunther, N. W., V. Lockatell, D. E. Johnson, and H. L. Mobley. 2001. In vivo dynamics of type 1 fimbria regulation in uropathogenic Escherichia coli during experimental urinary tract infection. Infect. Immun. 69:2838-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagberg, L., R. Hull, S. Hull, S. Falkow, R. Freter, and E. C. Svanborg. 1983. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect. Immun. 40:265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagberg, L., I. Engberg, R. Freter, J. Lam, S. Olling, and E. C. Svanborg. 1983. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun. 40:273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedlund, M., B. Frendeus, C. Wachtler, L. Hang, H. Fischer, and C. Svanborg. 2001. Type 1 fimbriae deliver an LPS- and TLR4-dependent activation signal to CD14-negative cells. Mol. Microbiol. 39:542-552. [DOI] [PubMed] [Google Scholar]
- 17.Iwahi, T., Y. Abe, M. Nakao, A. Imada, and K. Tsuchiya. 1983. Role of type 1 fimbriae in the pathogenesis of ascending urinary tract infection induced by Escherichia coli in mice. Infect. Immun. 39:1307-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keith, B. R., L. Maurer, P. A. Spears, and P. E. Orndorff. 1986. Receptor-binding function of type 1 pili effects bladder colonization by a clinical isolate of Escherichia coli. Infect. Immun. 53:693-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kisielius, P. V., W. R. Schwan, S. K. Amundsen, J. L. Duncan, and A. J. Schaeffer. 1989. In vivo expression and variation of Escherichia coli type 1 and P pili in the urine of adults with acute urinary tract infections. Infect. Immun. 57:1656-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klemm, P. 1986. Two regulatory fim genes. fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 5:1389-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langermann, S., S. Palaszynski, M. Barnhart, G. Auguste, J. S. Pinkner, J. Burlein, P. Barren, S. Koenig, S. Leath, C. H. Jones, and S. J. Hultgren. 1997. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 276:607-611. [DOI] [PubMed] [Google Scholar]
- 22.Li, X., D. E. Johnson, and H. L. T. Mobley. 1999. Identification of MrpH as the MR/P hemagglutinin of uropathogenic Proteus mirabilis. Infect. Immun. 67:2822-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim, J. K., N. W. Gunther, H. Zhao, D. E. Johnson, S. K. Keay, and H. L. Mobley. 1998. In vivo phase variation of Escherichia coli type 1 fimbrial genes in women with urinary tract infection. Infect. Immun. 66:3303-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Mobley, H. L., D. M. Green, A. L. Trifillis, D. E. Johnson, G. R. Chippendale, C. V. Lockatell, B. D. Jones, and J. W. Warren. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58:1281-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mobley, H. L. T., K. G. Jarvis, J. P. Elwood, D. I. Whittle, C. V. Lockatell, R. G. Russell, D. E. Johnson, M. S. Donnenberg, and J. W. Warren. 1993. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of αGal(1-4)βGal binding in virulence of a wild-type strain. Mol. Microbiol. 10:143-155. [DOI] [PubMed] [Google Scholar]
- 27.Mulvey, M. A., Y. S. Lopez-Boado, C. L. Wilson, R. Roth, W. C. Parks, J. Heuser, and S. J. Hultgren. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494-1497. [DOI] [PubMed] [Google Scholar]
- 28.Nowicki, B., M. Rhen, V. Vaisanen-Rhen, A. Pere, and T. K. Korhonen. 1984. Immunofluorescence study of fimbrial phase variation in Escherichia coli KS71. J. Bacteriol. 160:691-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen, P. B., and P. Klemm. 1994. Localization of promoters in the fim gene cluster and the effect of H-NS on the transcription of fimB and fimE. FEMS Microbiol. Lett. 116:95-100. [DOI] [PubMed] [Google Scholar]
- 30.Schaeffer, A. J. 1991. Potential role of phase variation of type 1 pili in urinary tract infection. and bacterial prostatitis. Infection 19(Suppl. 3):S144-S149. [DOI] [PubMed] [Google Scholar]
- 31.Schembri, M. A., P. B. Olsen, and P. Klemm. 1998. Orientation-dependent enhancement by H-NS of the activity of the type 1 fimbrial phase switch promoter in Escherichia coli. Mol. Gen. Genet. 259:336-344. [DOI] [PubMed] [Google Scholar]
- 32.Steadman, R., N. Topley, D. E. Jenner, M. Davies, and J. D. Williams. 1988. Type 1 fimbriate Escherichia coli stimulates a unique pattern of degranulation by human polymorphonuclear leukocytes. Infect. Immun. 56:815-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Struve, C., and K. A. Krogfelt. 1999. In vivo detection of Escherichia coli type 1 fimbrial expression and phase variation during experimental urinary tract infection. Microbiology 145:2683-2690. [DOI] [PubMed] [Google Scholar]
- 34.Svanborg, C., B. Frendeus, G. Godaly, L. Hang, M. Hedlund, and C. Wachtler. 2001. Toll-like receptor signaling and chemokine receptor expression influence the severity of urinary tract infection. J. Infect. Dis. 183(Suppl. 1):S61-S65. [DOI] [PubMed] [Google Scholar]
- 35.Xia, Y., D. Gally, K. Forsman-Semb, and B. E. Uhlin. 2000. Regulatory cross-talk between adhesin operons in Escherichia coli: inhibition of type 1 fimbriae expression by the PapB protein. EMBO J. 19:1450-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao, H., X. Li, D. E. Johnson, I. Blomfield, and H. L. Mobley. 1997. In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilis infecting the urinary tract. Mol. Microbiol. 23:1009-1019. [DOI] [PubMed] [Google Scholar]