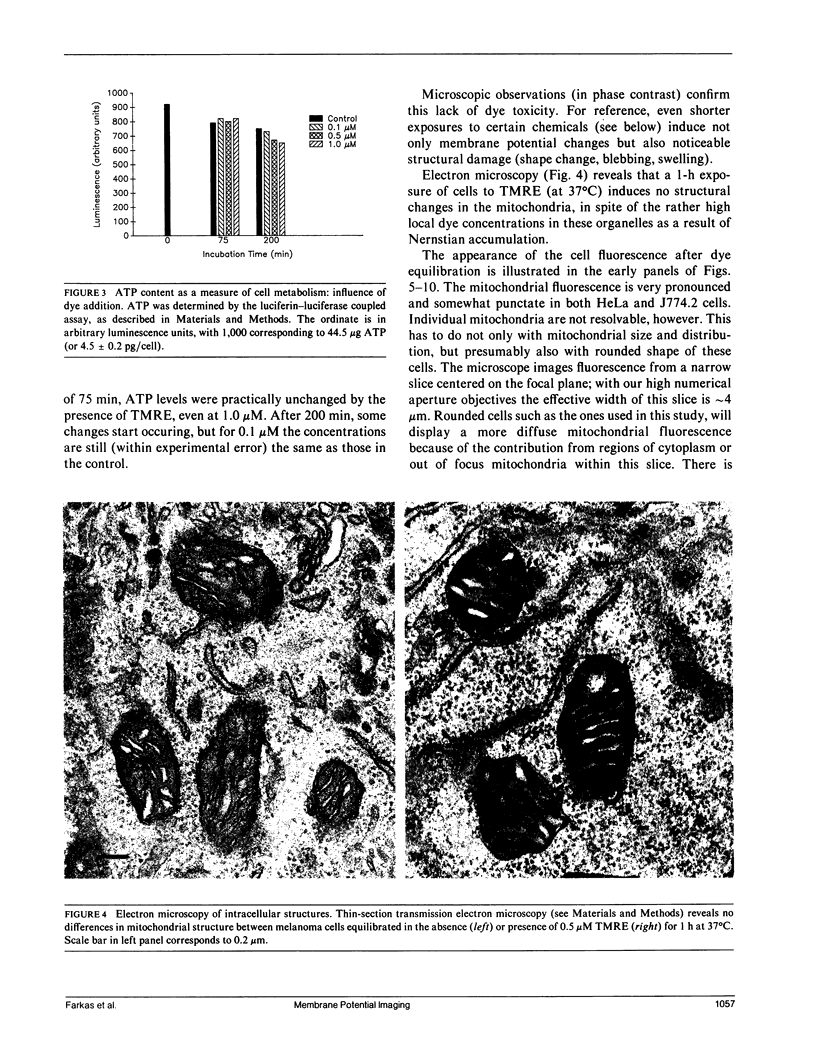
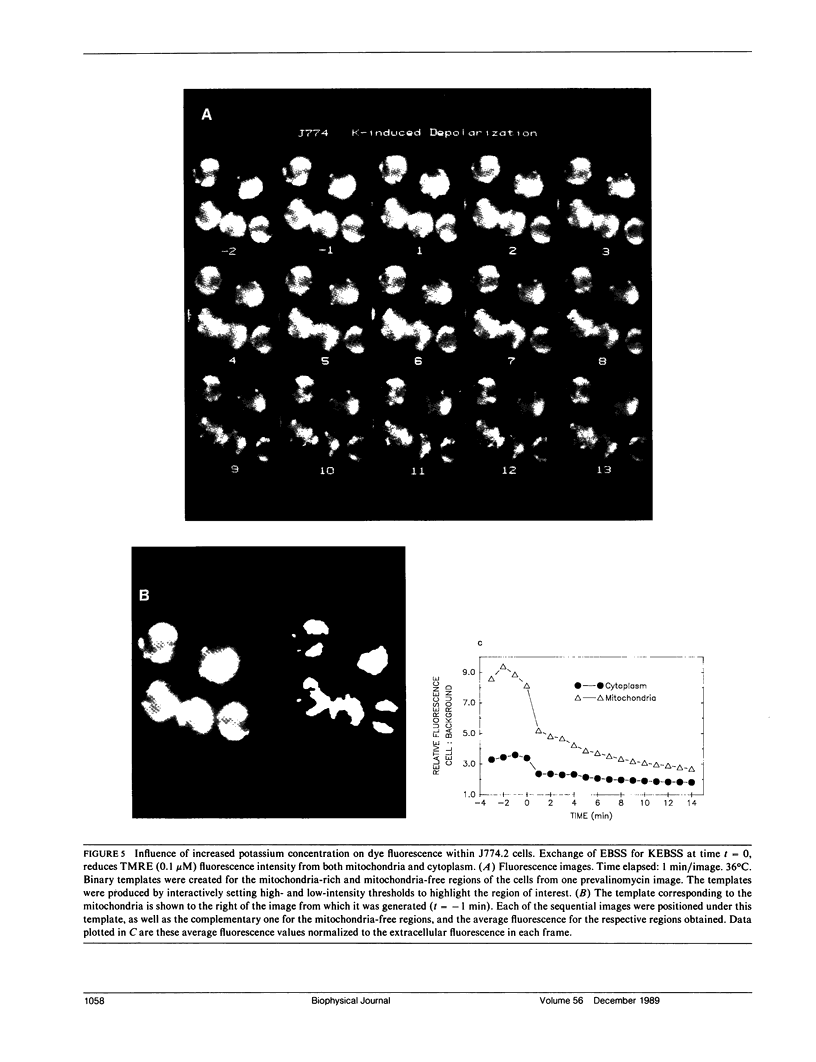
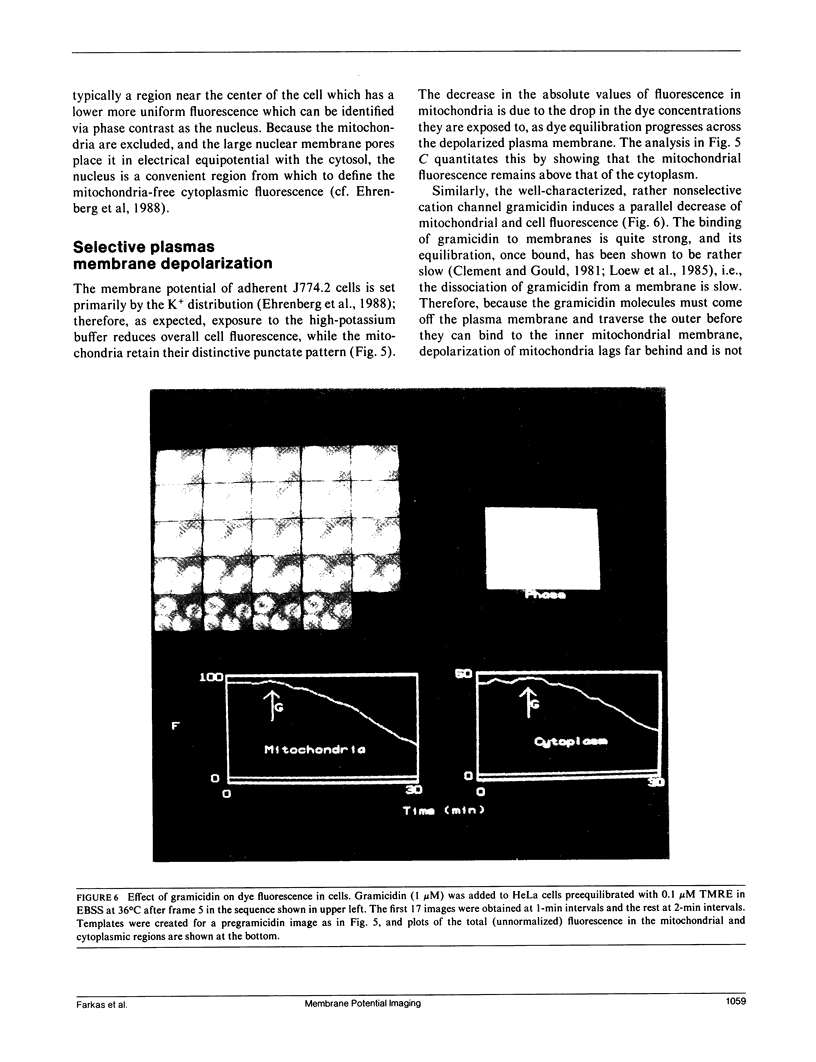
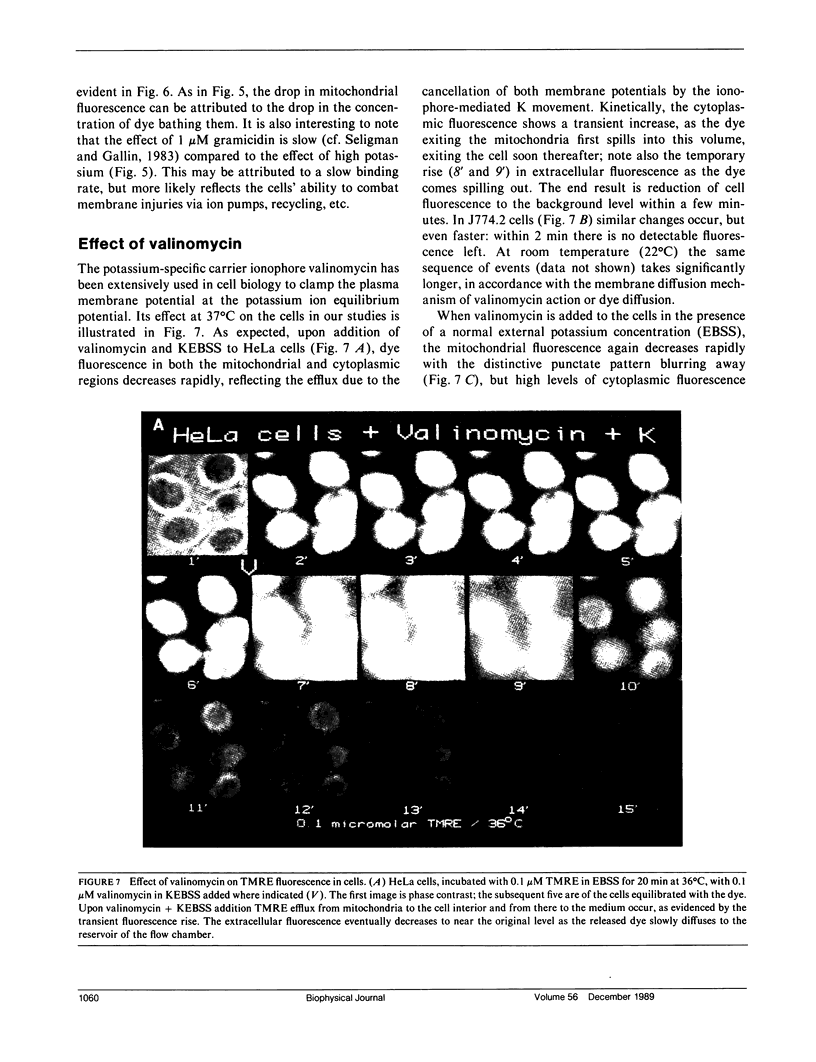
Abstract
The distribution of charged membrane-permeable molecular probes between intracellular organelles, the cytoplasm, and the outside medium is governed by the relative membrane electrical potentials of these regions through coupled equilibria described by the Nernst equation. A series of highly fluorescent cationic dyes of low membrane binding and toxicity (Ehrenberg, B., V. Montana, M.-D. Wei, J. P. Wuskell, and L. M. Loew, 1988. Biophys. J. 53:785-794) allows the monitoring of these equilibria through digital imaging video microscopy. We employ this combination of technologies to assess, simultaneously, the membrane potentials of cells and of their organelles in situ. We describe the methodology and optimal conditions for such measurements, and apply the technique to concomitantly follow, with good time resolution, the mitochondrial and plasma membrane potentials in several cultured cell lines. The time course of variations induced by chemical agents (ionophores, uncouplers, electron transport, and energy transfer inhibitors) in either or both these potentials is easily quantitated, and in accordance with mechanistic expectations. The methodology should therefore be applicable to the study of more subtle and specific, biologically induced potential changes in cells.
Full text
PDF



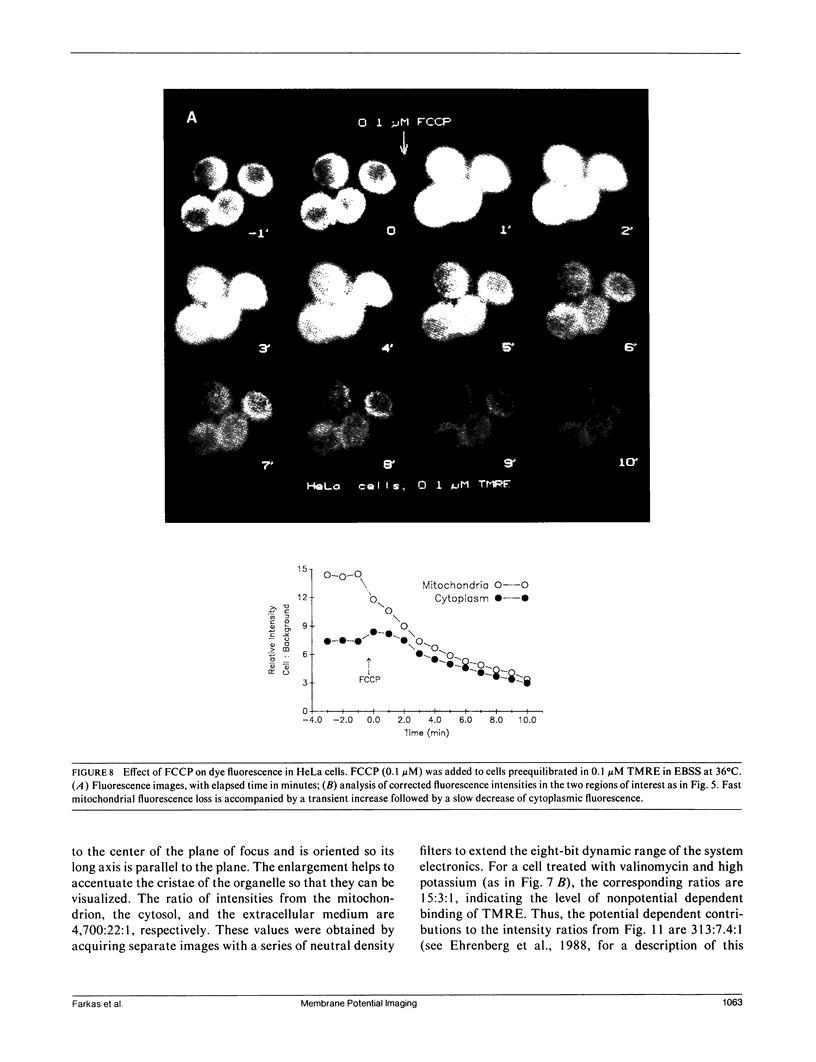
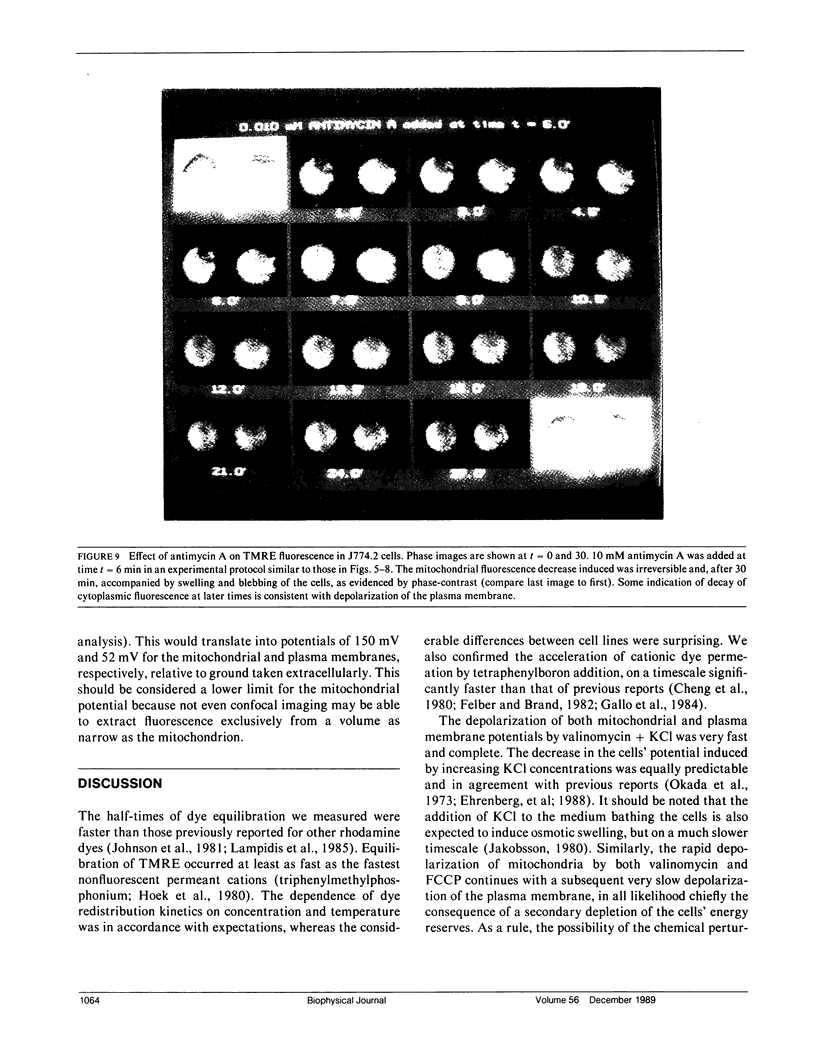
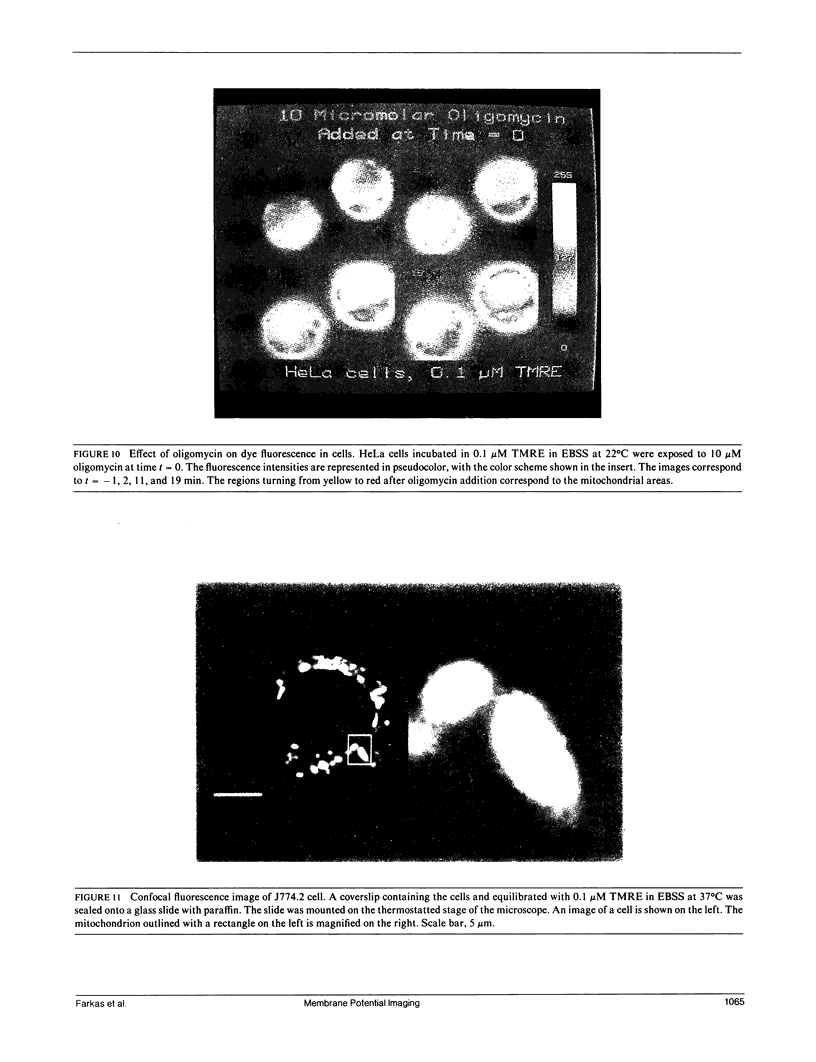




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A. Optical sectioning microscopy: cellular architecture in three dimensions. Annu Rev Biophys Bioeng. 1984;13:191–219. doi: 10.1146/annurev.bb.13.060184.001203. [DOI] [PubMed] [Google Scholar]
- Andersen O. S. Gramicidin channels. Annu Rev Physiol. 1984;46:531–548. doi: 10.1146/annurev.ph.46.030184.002531. [DOI] [PubMed] [Google Scholar]
- Barrows G. H., Sisken J. E., Allegra J. C., Grasch S. D. Measurement of fluorescence using digital integration of video images. J Histochem Cytochem. 1984 Jul;32(7):741–746. doi: 10.1177/32.7.6736626. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Alder G. M., Gray M. A., Micklem K. J., Taylor C. C., Turek P. J., Pasternak C. A. Oxonol dyes as monitors of membrane potential: the effect of viruses and toxins on the plasma membrane potential of animal cells in monolayer culture and in suspension. J Cell Physiol. 1985 Jun;123(3):326–336. doi: 10.1002/jcp.1041230306. [DOI] [PubMed] [Google Scholar]
- Benson D. M., Bryan J., Plant A. L., Gotto A. M., Jr, Smith L. C. Digital imaging fluorescence microscopy: spatial heterogeneity of photobleaching rate constants in individual cells. J Cell Biol. 1985 Apr;100(4):1309–1323. doi: 10.1083/jcb.100.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter-Hahn J., Seipel K. H., Vöth M., Ploem J. S. Fluorimetry of mitochondria in cells vitally stained with DASPMI or rhodamine 6 GO. Cell Biochem Funct. 1983 Oct;1(3):147–155. doi: 10.1002/cbf.290010306. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Block S. M. A miniature flow cell designed for rapid exchange of media under high-power microscope objectives. J Gen Microbiol. 1984 Nov;130(11):2915–2920. doi: 10.1099/00221287-130-11-2915. [DOI] [PubMed] [Google Scholar]
- Cheng K., Haspel H. C., Vallano M. L., Osotimehin B., Sonenberg M. Measurement of membrane potentials (psi) of erythrocytes and white adipocytes by the accumulation of triphenylmethylphosphonium cation. J Membr Biol. 1980 Oct 31;56(3):191–201. doi: 10.1007/BF01869476. [DOI] [PubMed] [Google Scholar]
- Clement N. R., Gould J. M. Kinetics for development of gramicidin-induced ion permeability in unilamellar phospholipid vesicles. Biochemistry. 1981 Mar 17;20(6):1544–1548. doi: 10.1021/bi00509a021. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M. Optical measurement of membrane potential. Rev Physiol Biochem Pharmacol. 1978;83:35–88. doi: 10.1007/3-540-08907-1_2. [DOI] [PubMed] [Google Scholar]
- Cohen R. L., Muirhead K. A., Gill J. E., Waggoner A. S., Horan P. K. A cyanine dye distinguishes between cycling and non-cycling fibroblasts. Nature. 1981 Apr 16;290(5807):593–595. doi: 10.1038/290593a0. [DOI] [PubMed] [Google Scholar]
- DeBiasio R., Bright G. R., Ernst L. A., Waggoner A. S., Taylor D. L. Five-parameter fluorescence imaging: wound healing of living Swiss 3T3 cells. J Cell Biol. 1987 Oct;105(4):1613–1622. doi: 10.1083/jcb.105.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg B., Montana V., Wei M. D., Wuskell J. P., Loew L. M. Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes. Biophys J. 1988 May;53(5):785–794. doi: 10.1016/S0006-3495(88)83158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emaus R. K., Grunwald R., Lemasters J. J. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta. 1986 Jul 23;850(3):436–448. doi: 10.1016/0005-2728(86)90112-x. [DOI] [PubMed] [Google Scholar]
- Felber S. M., Brand M. D. Factors determining the plasma-membrane potential of lymphocytes. Biochem J. 1982 May 15;204(2):577–585. doi: 10.1042/bj2040577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman J. C., Laris P. C. Electrophysiology of cells and organelles: studies with optical potentiometric indicators. Int Rev Cytol Suppl. 1981;12:177–246. doi: 10.1016/b978-0-12-364373-5.50015-9. [DOI] [PubMed] [Google Scholar]
- Gallo R. L., Finkelstein J. N., Notter R. H. Characterization of the plasma and mitochondrial membrane potentials of alveolar type II cells by the use of ionic probes. Biochim Biophys Acta. 1984 Apr 11;771(2):217–227. doi: 10.1016/0005-2736(84)90536-4. [DOI] [PubMed] [Google Scholar]
- Gear A. R. Rhodamine 6G. A potent inhibitor of mitochondrial oxidative phosphorylation. J Biol Chem. 1974 Jun 10;249(11):3628–3637. [PubMed] [Google Scholar]
- Higuti T., Niimi S., Saito R., Nakasima S., Ohe T., Tani I., Yoshimura T. Rhodamine 6G, inhibitor of both H+-ejections from mitochondria energized with ATP and with respiratory substrates. Biochim Biophys Acta. 1980 Dec 3;593(2):463–467. doi: 10.1016/0005-2728(80)90081-x. [DOI] [PubMed] [Google Scholar]
- Hoek J. B., Nicholls D. G., Williamson J. R. Determination of the mitochondrial protonmotive force in isolated hepatocytes. J Biol Chem. 1980 Feb 25;255(4):1458–1464. [PubMed] [Google Scholar]
- Jakobsson E. Interactions of cell volume, membrane potential, and membrane transport parameters. Am J Physiol. 1980 May;238(5):C196–C206. doi: 10.1152/ajpcell.1980.238.5.C196. [DOI] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Bockus B. J., Chen L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol. 1981 Mar;88(3):526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H. M., Rich A. M., Wilkenfeld C., Rutherford L. E., Weissmann G. A carbocyanine dye, DiOC6(3), acts as a mitochondrial probe in human neutrophils. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1495–1501. doi: 10.1016/s0006-291x(82)80076-4. [DOI] [PubMed] [Google Scholar]
- Lampidis T. J., Hasin Y., Weiss M. J., Chen L. B. Selective killing of carcinoma cells "in vitro" by lipophilic-cationic compounds: a cellular basis. Biomed Pharmacother. 1985;39(5):220–226. [PubMed] [Google Scholar]
- Leach F. R., Webster J. J. Commercially available firefly luciferase reagents. Methods Enzymol. 1986;133:51–70. doi: 10.1016/0076-6879(86)33055-6. [DOI] [PubMed] [Google Scholar]
- Lichtshtein D., Kaback H. R., Blume A. J. Use of a lipophilic cation for determination of membrane potential in neuroblastoma-glioma hybrid cell suspensions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):650–654. doi: 10.1073/pnas.76.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnett P. E., Beechey R. B. Inhibitors of the ATP synthethase system. Methods Enzymol. 1979;55:472–518. doi: 10.1016/0076-6879(79)55061-7. [DOI] [PubMed] [Google Scholar]
- Loew L. M., Benson L., Lazarovici P., Rosenberg I. Fluorometric analysis of transferable membrane pores. Biochemistry. 1985 Apr 23;24(9):2101–2104. doi: 10.1021/bi00330a001. [DOI] [PubMed] [Google Scholar]
- Lundin A., Hasenson M., Persson J., Pousette A. Estimation of biomass in growing cell lines by adenosine triphosphate assay. Methods Enzymol. 1986;133:27–42. doi: 10.1016/0076-6879(86)33053-2. [DOI] [PubMed] [Google Scholar]
- Mai M. S., Allison W. S. Inhibition of an oligomycin-sensitive ATPase by cationic dyes, some of which are atypical uncouplers of intact mitochondria. Arch Biochem Biophys. 1983 Mar;221(2):467–476. doi: 10.1016/0003-9861(83)90165-0. [DOI] [PubMed] [Google Scholar]
- Mokhova E. N., Rozovskaya I. A. The effects of mitochondrial energetics inhibitors on the fluorescence of potential-sensitive dyes rhodamine 123 and diS-C3-(5) in lymphocyte suspensions. J Bioenerg Biomembr. 1986 Aug;18(4):265–276. doi: 10.1007/BF00743047. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Okada Y., Ogawa M., Aoki N., Izutsu K. The effect of K + on the membrane potential in HeLa cells. Biochim Biophys Acta. 1973 Jan 2;291(1):116–126. doi: 10.1016/0005-2736(73)90066-7. [DOI] [PubMed] [Google Scholar]
- Preston S. F., Volpi M., Pearson C. M., Berlin R. D. Regulation of cell shape in the Cloudman melanoma cell line. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5247–5251. doi: 10.1073/pnas.84.15.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes J., Benos D. J. Changes in interfacial potentials induced by carbonylcyanide phenylhydrazone uncouplers: possible role in inhibition of mitochondrial oxygen consumption and other transport processes. Membr Biochem. 1984;5(3):243–268. doi: 10.3109/09687688409150281. [DOI] [PubMed] [Google Scholar]
- Ritchie R. J. A critical assessment of the use of lipophilic cations as membrane potential probes. Prog Biophys Mol Biol. 1984;43(1):1–32. doi: 10.1016/0079-6107(84)90002-6. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Scott I. D., Nicholls D. G. Energy transduction in intact synaptosomes. Influence of plasma-membrane depolarization on the respiration and membrane potential of internal mitochondria determined in situ. Biochem J. 1980 Jan 15;186(1):21–33. doi: 10.1042/bj1860021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligmann B. E., Gallin J. I. Comparison of indirect probes of membrane potential utilized in studies of human neutrophils. J Cell Physiol. 1983 May;115(2):105–115. doi: 10.1002/jcp.1041150202. [DOI] [PubMed] [Google Scholar]
- Singer T. P. Mitochondrial electron-transport inhibitors. Methods Enzymol. 1979;55:454–462. doi: 10.1016/0076-6879(79)55059-9. [DOI] [PubMed] [Google Scholar]
- Sung S. S., Young J. D., Origlio A. M., Heiple J. M., Kaback H. R., Silverstein S. C. Extracellular ATP perturbs transmembrane ion fluxes, elevates cytosolic [Ca2+], and inhibits phagocytosis in mouse macrophages. J Biol Chem. 1985 Nov 5;260(25):13442–13449. [PubMed] [Google Scholar]
- White J. G., Amos W. B., Fordham M. An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J Cell Biol. 1987 Jul;105(1):41–48. doi: 10.1083/jcb.105.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieker H. J., Kuschmitz D., Hess B. Inhibition of yeast mitochondrial F1-ATPase, F0F1-ATPase and submitochondrial particles by rhodamines and ethidium bromide. Biochim Biophys Acta. 1987 Jun 9;892(1):108–117. doi: 10.1016/0005-2728(87)90253-2. [DOI] [PubMed] [Google Scholar]
- Yaginuma N., Hirose S., Inada Y. Spectral change of rhodamine 6G caused by the energization of mitochondria, in relation to charge separation. J Biochem. 1973 Oct;74(4):811–815. doi: 10.1093/oxfordjournals.jbchem.a130307. [DOI] [PubMed] [Google Scholar]