Abstract
We show here that Mg2+ acquisition by CorA is essential for Helicobacter pylori in vitro, as corA mutants did not grow in media without Mg2+ supplementation. Complementation analysis performed with an Escherichia coli corA mutant revealed that H. pylori CorA transports nickel and cobalt in addition to Mg2+. However, Mg2+ is the dominant CorA substrate, as the corA mutation affected neither cobalt and nickel resistance nor nickel induction of urease in H. pylori. The drastic Mg2+ requirement (20 mM) of H. pylori corA mutants indicates that CorA plays a key role in the adaptation to the low-Mg2+ conditions predominant in the gastric environment.
Magnesium (Mg2+) is a cofactor of many enzymes involved in central biochemical pathways which are essential for bacterial viability (20, 23). In order to overcome the Mg2+ limitation within the human host, pathogenic bacteria express specific Mg2+ uptake systems. In Salmonella enterica serovar Typhimurium and Escherichia coli, high-affinity Mg2+ uptake is mainly mediated by the inner membrane protein CorA, which also imports nickel and cobalt (16, 23). The E. coli CorA protein was shown to be involved in iron uptake (13). Enterobacteria have multiple Mg2+ uptake systems, and thus corA mutants display a reduced Mg2+ transport capacity as well as increased cobalt and nickel resistance (23). The cation metabolism of the gastric pathogen Helicobacter pylori (10), which causes various diseases of the upper gastrointestinal tract, is of substantial importance for survival in the hostile and changing environment of the gastric mucosa (18, 21). Mechanisms involved in maintaining cation homeostasis were shown to be required for effective gastric colonization in animal models (25). Although the essential biological functions of Mg2+ point towards a relevance of Mg2+ acquisition in the adaptation to the gastric environment, proteins involved in H. pylori Mg2+ uptake and metabolism have not been studied in detail. A single corA gene homolog (HP1344/JHP1263) was identified in the annotated genome sequences of H. pylori strains 26695 (24) and J99 (1). Furthermore, a possible nickel uptake function of H. pylori CorA might be of substantial importance because H. pylori expresses high amounts of the nickel metalloenzyme urease for neutralization of gastric acid (14, 18, 19). Nickel induces both urease expression and activity (26). Therefore, we investigated H. pylori CorA functions by analysis of corA mutants as well as by overexpression of the H. pylori corA gene in an E. coli corA mutant. For the experiments, H. pylori was routinely grown under microaerobic conditions as described earlier (6, 9). DNA manipulation and transformation were performed according to standard protocols for E. coli (2) and H. pylori (11). The complete corA gene (Fig. 1) was amplified by PCR from isolated DNA of H. pylori strain 26695 using primer oligonucleotides CORA-L1 (ATATAGGGCCTGCGAGCTTG) and -R1 (CGAGCGATCATAGCCAGACC) and cloned into plasmid pZERO-1 (Table 1). The resulting plasmids pCORA+ and pCORA− contain the H. pylori corA gene in and against the direction of the lacZ promoter present in the plasmid, respectively (Fig. 1; Table 1). For the growth experiments or the determinations of the MICs of Mg2+ and of various metals, E. coli and H. pylori were precultured to optical densities at 550 nm (OD550) and 600 nm (OD600) of 1.0 in Luria-Bertani (LB) and brucella broth (Oxoid) supplemented with 5% fetal calf serum (BBF; Gibco), respectively. For growth experiments, bacterial suspensions were subsequently diluted 1:100 in test media containing defined supplements.
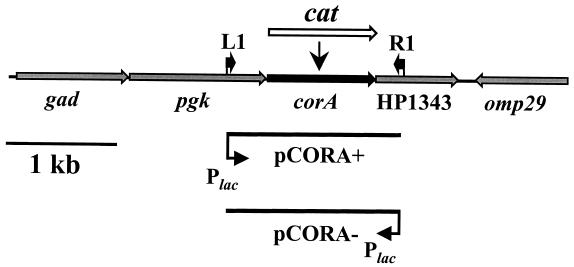
FIG. 1.
Genetic organization and mutagenesis of H. pylori corA with overview of the corA region in H. pylori strain 26695. Genes and primer binding sites are marked by gray and black arrows, respectively. The insertion site of the cat cassette (white arrow) is indicated. Genes are numbered according to the annotated genome sequence (24). The orientation of the DNA region cloned in plasmids pCORA+ and pCORA− with respect to the lacZ promoter (PlacZ) present in the cloning vector pZERO-1 is indicated.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source(s) |
|---|---|---|
| Strains | ||
| H. pylori | ||
| 1061 | wt, cag− | 5, 6, 8 |
| 1061-CORA1 | 1061, corA::cat, Cmr | This study |
| 26695 | wt, cag+ | 24 |
| 26695-CORA1 | 26695, corA::cat, Cmr | This study |
| 26695-CORA2 | 26695, corA::Pcat, Cmr | This study |
| E. coli | ||
| MC4100 | araD139 Δ(argF-lac)169 flhD5301, fruA25 relA1 rpsL150 rbsR22 deoC1 | 7 |
| H5324 | MC4100, corA::Tn10, Tetr | 13 |
| Plasmids | ||
| pZERO-1 | Cloning vector, MCS in lacZ′, zeo, Zeor | Invitrogen |
| pZERO-2 | Cloning vector, MCS in lacZ′, neo, Kmr | Invitrogen |
| pCORA+ | pZERO-1 with the corA gene of H. pylori strain 26695 cloned in the direction of the lacZ promoter, Zeor | This study |
| pCORA− | pZERO-1 with the corA gene of H. pylori strain 26695 cloned against the direction of the lacZ promoter, Zeor | This study |
| pCORA-CAT | pZERO-2 carrying a corA::cat fusion, Cmr | This study |
| pCORA-PCAT | pZERO-2 carrying a corA::Pcat fusion, Cmr | This study |
| pHel3 | H. pylori shuttle vector, neo, Kmr | 15 |
| pHel3-CORA | pHel3 without the E. coli origin of replication fused with pCORA+, Kmr Zeor | This study |
MCS, multiple cloning site; cag, cag pathogenicity island.
Isolation of H. pylori corA mutants in the presence of Mg2+.
For insertional inactivation of the H. pylori corA gene, we constructed the plasmids pCORA-PCAT and pCORA-CAT, which contain the chloramphenicol acetyltransferase gene cat with (Pcat) and without its own promoter inserted in the corA gene, respectively (Fig. 1; Table 1). The Pcat and cat sequences amplified by PCR with primers CAT-L1 (5′-TCCGGTTTTTGTTAATCCGCC) or CAT-L2 (TCCGAGATTTTCAGGAG) in combination with primer CAT-R1 (TTACGCCCCGCCCTGCCA), respectively, were fused to corA upstream and downstream DNA regions by megaprimer PCR (22). Therefore, corA flanking regions were amplified from DNA of H. pylori strain 26695 with primers carrying 5′ extensions complementary to the 5′ and 3′ ends of the Pcat and cat cassettes (underlined below), respectively. The PCR products generated with primer pairs CORA-L1 and CATCORA-R1 (CTCCTGAAAATCTCGGAAAGTCCCATAATAGATCGTG) or PCATCORA-L1 (GGCGGATTAACAAAAACCGGAAGTCCCATAATAGATCGTG) and CATCORA-L1 (TGGCAGGGCGGGGCGTAATGAAGACGGGTTTGATATTC)/CORA-R1 were purified and subsequently mixed with PCR-amplified Pcat or cat cassettes to work as megaprimers in a second PCR containing the flanking primers CORA-L1 and -R1. Megaprimer PCR products carrying Pcat or cat inserted into the corA gene were cloned into plasmid pZERO-2 (Invitrogen), resulting in plasmids pCORA-PCAT and pCORA-CAT, respectively (Fig. 1). Due to the pronounced interstrain variability of H. pylori we analyzed corA functions in H. pylori strains 26695 and 1061, which contain (24) and lack (8) the entire cag pathogenicity island, respectively (Table 1). To achieve inactivation of corA by site-directed mutagenesis, the plasmids pCORA-PCAT and pCORA-CAT (Table 1; Fig. 1) were introduced into H. pylori strains 26695 and 1061 by electroporation and by natural transformation, respectively. The fact that repeated transformation and subsequent selection for transformants did not result in colonies displaying a Cmr phenotype provided evidence that the corA gene is essential for H. pylori viability in vitro. To avoid a possible killing of corA mutants by Mg2+ depletion, we supplemented the Dent agar with 20 mM Mg2+ (catalog no. 105833; Merck). Under these conditions, H. pylori corA::cat and corA::Pcat mutants could be selected. After PCR analysis revealed correct insertion of the cat and Pcat cassettes in the corA gene, these mutants (Table 1) were further investigated.
Determination of the Mg2+ requirement of H. pylori corA mutants.
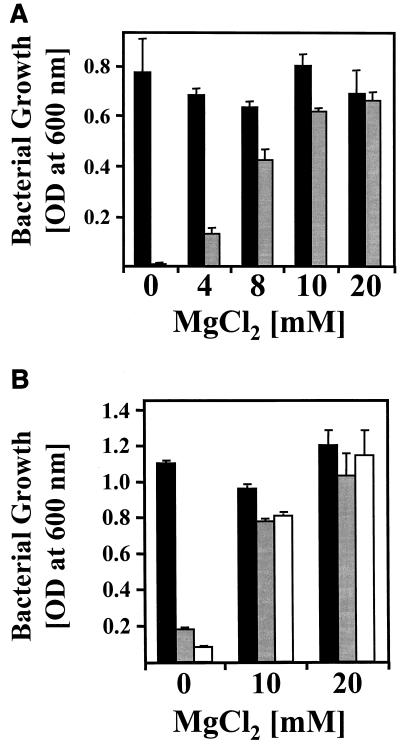
The Mg2+ requirement caused by the corA mutation was further investigated by growth experiments. The corA mutants of both H. pylori strains did not grow in unsupplemented broth, indicating that the Mg2+ concentration of 1.7 mM in BBF (determined with a Sigma kit; catalog no. 595-M) is not sufficient to restore Mg2+ acquisition (Fig. 2). Supplementation of BBF with 20 mM MgCl2 enabled the corA mutants to grow, whereas CaCl2 and NaCl added at identical concentrations as controls had no effect (data not shown). Precultivation in BBF with 20 mM Mg2+ and subsequent monitoring of growth in BBF with defined Mg2+ concentrations revealed that the corA mutation causes in both H. pylori strains 1061 and 26695 a drastic Mg2+ requirement in the range of 20 mM (Fig. 2). The strong promoter in front of the cat gene had no effect on the growth behavior of the H. pylori 26695 corA mutants (Fig. 2B), providing evidence that the Mg2+ requirement is caused by the corA mutation and not by polar effects on the downstream genes (Fig. 1). However, as the corA gene is localized in an operon-like structure (Fig. 1), possible polar effects on neighboring genes were further excluded by complementation of the corA mutation with the intact corA gene cloned in the H. pylori plasmid pHel3 (Table 1). If introduced into the corA mutant of strain 1061, the resulting plasmid pHel3-CORA enabled the transformants to grow on Dent agar without Mg2+ supplementation.
FIG. 2.
Determination of the Mg2+ requirement of H. pylori corA mutants. (A) Mg2+ requirement caused by the corA mutation in H. pylori strain 1061. The growth of H. pylori strain 1061 (black bars) and of the corA mutant 1061-CORA1 (gray bars) in BBF was determined by measuring the OD600. Bacteria were precultured to an OD600 of 1.0 in BBF medium with 20 mM Mg2+ and then subcultured in BBF supplemented with Mg2+ at increasing concentrations, indicated on the x axis. (B) Mg2+ requirement caused by the corA mutation in H. pylori strain 26695. The growth of H. pylori strains 26695 (black bars), 26695-CORA1 (corA::cat; gray bars) and 26695-CORA2 (corA::Pcat; white bars) was determined by measuring the OD600. Bacteria were precultured to an OD600 of 1.0 in BBF medium with 20 mM Mg2+ and then subcultured in BBF supplemented with Mg2+ at increasing concentrations, indicated on the x axis. The data shown represent mean values from three independent determinations. Standard deviations are indicated.
Analysis of Mg2+ and metal transport functions of H. pylori CorA in E. coli.
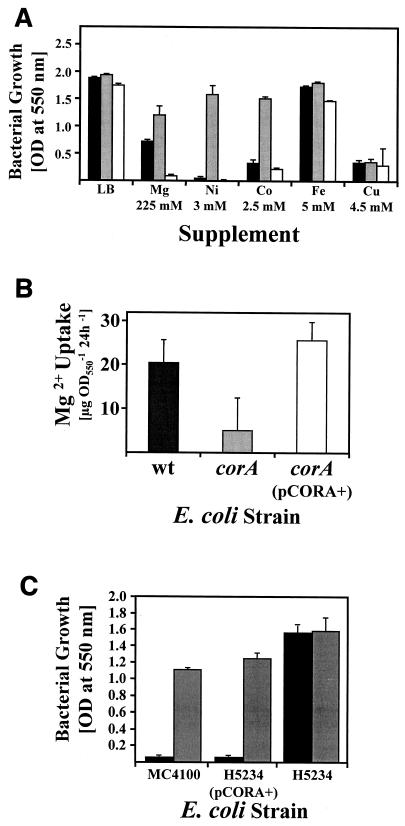
The analysis of CorA transport functions in H. pylori was restricted by the drastic Mg2+ requirement of the corA mutants. Therefore, we studied functions of H. pylori CorA in Mg2+ and metal uptake in the corA mutant H5324 of the E. coli strain MC4100 (wild type [wt]) (Table 1). Strain H5324 displays classical corA phenotypes, namely, increased resistance to cobalt, nickel, and Mg2+, as determined in preliminary growth experiments (Fig. 3A). Cation concentrations toxic for the parental strain but not for the corA mutant were determined by growth inhibition experiments in LB medium supplemented with Mg2+, nickel (catalog no. N5756; Sigma), cobalt (catalog no. 2539; Merck), iron (catalog no. F2877; Sigma), and copper (catalog no. C6641; Sigma) at increasing concentrations. Under these growth conditions a restoration of E. coli H5324 metal sensitivity indicated CorA-mediated cation import (Fig. 3A). The subsequent analysis of corA phenotypes in strain H5324 transformants carrying intact versions of H. pylori corA on plasmids pCORA+ and pCORA− (Table 1) showed that plasmid pCORA+ restores Mg2+, nickel, and cobalt sensitivity of E. coli H5324 to wild-type (wt) levels (Fig. 3A). Similar to CorA of enterobacteria, H. pylori CorA mediates transport of all three elements. Copper resistance was not influenced by CorA. After 12 h of growth, the iron resistance of strain H5324(pCORA+) was reduced to 50% of that of strain H5324. This transient effect, which was not observed after 24 h of growth (Fig. 3A), suggests that CorA mediates low-affinity iron transport, as proposed earlier (27). The plasmid pCORA− did not suppress the corA-mediated phenotypes of E. coli H5324, providing evidence that the expression of the H. pylori corA gene in plasmid pCORA+ is driven by the lacZ promoter (Fig. 1). Determinations of the residual Mg2+ content of the media after bacterial growth revealed that H. pylori corA does completely restore the Mg2+ transport defect in E. coli H5324 (Fig. 3B). Furthermore, additive supplementation with Mg2+ in equimolar concentrations abolished the nickel and cobalt sensitivities of strain H5324(pCORA+), indicating that Mg2+ is the dominant substrate of H. pylori CorA (data not shown). Finally, nickel-hexaamine (Ni-HA), a potent inhibitor of S. enterica serovar Typhimurium CorA (17), also affected E. coli and H. pylori CorA transport functions, as additive Ni-HA supplementation of media abolished nickel sensitivity of the E. coli strains MC4100 and H5324(pCORA+) in a concentration-dependent manner (Fig. 3C).
FIG. 3.
Analysis of H. pylori corA transport functions in the E. coli corA mutant H5324. (A) Sensitivity to Mg2+ and to various metals mediated by H. pylori corA in E. coli. The growth of E. coli strains MC4100 (black bars), H5324 (gray bars), and H5324 with plasmid pCORA+ carrying H. pylori corA (white bars) in the presence of Mg2+, nickel (Ni), cobalt (Co), iron (Fe), and copper (Cu) was determined by measuring the OD550. The Mg2+ and metal concentrations which were toxic for the parental strain but not for the corA mutant are indicated on the x axis. The data represent mean values from three independent determinations. Standard deviations are indicated. (B) Determination of Mg2+ uptake mediated by H. pylori CorA. The E. coli strains MC4100 (black bars), H5324 (gray bars), and H5324(pCORA+) carrying the H. pylori corA gene (white bars) grown for 24 h in LB medium were removed by centrifugation and the Mg2+ concentrations in the supernatants were determined. Uptake of Mg2+ was calculated from the Mg2+ concentration in the LB medium prior to inoculation. The data represent mean values from two independent determinations. Standard deviations are indicated. (C) The influence of Ni-HA onCorA-mediated nickel transport. The E. coli strains MC4100, H5324(pCORA+), and H5324 were grown in LB medium supplemented with 3.5 mM nickel in the absence (black bars) or presence (shaded bars) of 2 mM Ni-HA. Growth was monitored by measuring the OD550. The growth of the E. coli corA mutant, which was not affected by nickel or by the hexaamine, is shown as a control. The data represent mean values from three independent determinations. Standard deviations are indicated.
Influence of CorA on cobalt and nickel metabolism in H. pylori.
To test if CorA-mediated nickel and cobalt uptake contributes to H. pylori metal metabolism, we determined the MICs of both metals. The results of growth inhibition experiments in broth revealed that the corA mutation had no effect on the nickel and cobalt MICs of 3 mM and 20 μM, respectively, in strain 26695, indicating that CorA is not involved in maintaining metal homeostasis. The possible effect of corA on nickel metabolism was further investigated by analysis of urease expression and activity. Immunoblot analysis with the UreA-specific monoclonal antibody HPM-5021-5 (IBT, Reutlingen, Germany) revealed that neither basal expression nor nickel induction of H. pylori urease were influenced by Mg2+ supplementation or by the corA mutation (results not shown). Similar results were obtained for urease activity, which was determined with the Berthelot reaction as described earlier (3, 4, 26). After growth in BBF medium supplemented with 20 mM Mg2+ and 10 μm NiCl2 the H. pylori strains 26695 and 26695-CORA1 displayed nearly identical urease activities of 48.7 ± 2.7 and 49.3 ± 7.8 U in the wt strain and in the corA mutant, respectively, indicating that the corA mutation does not influence H. pylori nickel metabolism.
Conclusions.
The complete growth deficiency in media without Mg2+ supplementation and the drastic Mg2+ requirement in the range of 20 mM displayed by corA mutants demonstrates that H. pylori CorA is essential for Mg2+ acquisition required for survival in low-Mg2+ environments. These findings underline the role of H. pylori cation metabolism in maintaining metabolic functions and highlight for the first time a substantial importance of Mg2+ acquisition in gastric adaptation. A role of CorA-mediated Mg2+ uptake in H. pylori colonization and/or survival in the gastric mucosa is supported by the Mg2+ concentration in human gastric juice, which at 0.7 mM (12) is far below the values required for growth of corA mutants. Thus, it seems very unlikely that H. pylori corA mutants can persist in the gastric mucosa for extended time periods. The observation that neither Mg2+ supplementation nor the corA mutation influenced H. pylori cobalt and nickel resistance indicates that Mg2+ is the dominant CorA substrate. This suggests that H. pylori Mg2+ acquisition and metal ion uptake functions are strictly separated. This was further supported by the observations that additive Mg2+ supplementation of growth media completely inhibited nickel and cobalt sensitivity mediated by H. pylori CorA in E. coli, and that Mg2+ at concentrations as high as 20 mM did not influence either metal resistance or induction of urease by nickel (J. Pfeiffer and S. Bereswill, unpublished observations). The separation of metal and Mg2+ uptake functions prevents a competitive inhibition of nickel and cobalt uptake by Mg2+, as in most natural environments Mg2+ is about 102- to 103-fold more abundant than the trace metals. Finally, the lack of alternative Mg2+ uptake system homologs (23) in H. pylori (1, 24) and the absence of CorA homologs in eukaryotes (23) offer the possibility of using CorA as a target for the development of new H. pylori-specific drugs. The corA phenotypes observed suggest that the corA mutation could be used as a marker for genetic manipulation and, similar to essential genes in S. enterica serovar Typhimurium (28), they might facilitate the construction of H. pylori security strains for the production of live vaccines.
Acknowledgments
This work was financially supported by grant Ki201/9-1 from the Deutsche Forschungsgemeinschaft to M.K. and by a grant from Byk Gulden (Konstanz, Germany) to S.B.
We thank Tanja Vey for excellent technical assistance. The E. coli corA mutant H5324 was kindly provided by Klaus Hantke (University of Tübingen, Tübingen, Germany).
J.P. and J.G. contributed equally to this work.
Editor: J. T. Barbieri
REFERENCES
- 1.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1992. Short protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 3.Bereswill, S., S. Greiner, A. H. van Vliet, B. Waidner, F. Fassbinder, E. Schiltz, J. G. Kusters, and M. Kist. 2000. Regulation of ferritin-mediated cytoplasmic iron storage by the ferric uptake regulator homolog (Fur) of Helicobacter pylori. J. Bacteriol. 182:5948-5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bereswill, S., U. Waidner, S. Odenbreit, F. Lichte, F. Fassbinder, G. Bode, and M. Kist. 1998. Structural, functional and mutational analysis of the pfr gene encoding a ferritin from Helicobacter pylori. Microbiology 144:2505-2516. [DOI] [PubMed] [Google Scholar]
- 5.Bijlsma, J. J., C. M. J. E. Vandenbroucke-Grauls, S. H. Phadnis, and J. G. Kusters. 1999. Identification of virulence genes of Helicobacter pylori by random insertion mutagenesis. Infect. Immun. 67:2433-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bijlsma, J. J., B. Waidner, A. H. Vliet, N. J. Hughes, S. Hag, S. Bereswill, D. J. Kelly, C. M. J. E. Vandenbroucke-Grauls, M. Kist, and J. G. Kusters. 2002. The Helicobacter pylori homologue of the ferric uptake regulator is involved in acid resistance. Infect. Immun. 70:606-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casadaban, M. J. 1976. Regulation of the regulatory gene for the arabinose pathway, araC. J. Mol. Biol. 104:557-566. [DOI] [PubMed] [Google Scholar]
- 8.de Jonge, R., J. G. Kusters, M. S. Timmer, V. Gimmel, B. J. Appelmelk, S. Bereswill, A. H. M. van Vliet, S. G. Meuwissen, M. Kist, C. M. J. E. Vandenbroucke-Grauls, and E. J. Kuipers. 2001. The role of Helicobacter pylori virulence factors in interleukin production by monocytic cells. FEMS Microbiol. Lett. 196:235-238. [DOI] [PubMed] [Google Scholar]
- 9.Dent, J. C., and C. A. McNulty. 1988. Evaluation of a new selective medium for Campylobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 7:555-558. [DOI] [PubMed] [Google Scholar]
- 10.Dunn, B. E., H. Cohen, and M. J. Blaser. 1997. Helicobacter pylori. Clin. Microbiol. Rev. 10:720-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge, Z., and D. E. Taylor. 1997. Helicobacter pylori DNA transformation by natural competence and electroporation, p. 145-152. In C. L. Clayton and H. L. Mobley (ed.), Helicobacter pylori protocols. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 12.Greger, R., and U. Windhorst (ed.). 1996. Comprehensive human physiology, p. 2432. Springer, New York, N.Y.
- 13.Hantke, K. 1997. Ferrous iron uptake by a magnesium transport system is toxic for Escherichia coli and Salmonella typhimurium. J. Bacteriol. 179:6201-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawtin, P. R., H. T. Delves, and D. G. Newell. 1991. The demonstration of nickel in the urease of Helicobacter pylori by atomic absorption spectroscopy. FEMS Microbiol. Lett. 61:51-54. [DOI] [PubMed] [Google Scholar]
- 15.Heuermann, D., and R. Haas. 1998. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol. Gen. Genet. 257:519-528. [DOI] [PubMed] [Google Scholar]
- 16.Kehres, D. G., C. H. Lawyer, and M. E. Maguire. 1998. The CorA magnesium transporter gene family. Microb. Comp. Genomics 3:151-169. [DOI] [PubMed] [Google Scholar]
- 17.Kucharski, L. M., W. J. Lubbe, and M. E. Maguire. 2000. Cation hexaammines are selective and potent inhibitors of the CorA magnesium transport system. J. Biol. Chem. 275:16767-16773. [DOI] [PubMed] [Google Scholar]
- 18.McGee, D. J., and H. L. Mobley. 1999. Mechanisms of Helicobacter pylori infection: bacterial factors. Curr. Top. Microbiol. Immunol. 241:155-180. [DOI] [PubMed] [Google Scholar]
- 19.Mobley, H. L., M. D. Island, and R. P. Hausinger. 1995. Molecular biology of microbial ureases. Microbiol. Rev. 59:451-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moncrief, M. B., and M. E. Maguire. 1999. Magnesium transport in prokaryotes. J. Biol. Inorg. Chem. 4:523-527. [DOI] [PubMed] [Google Scholar]
- 21.Nolan, K. J., D. J. McGee, H. M. Mitchell, T. Kolesnikow, J. M. Harro, J. O'Rourke, J. E. Wilson, S. J. Danon, N. D. Moss, H. L. Mobley, and A. Lee. 2002. In vivo behavior of a Helicobacter pylori SS1 nixA mutant with reduced urease activity. Infect. Immun. 70:685-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar, G., and S. S. Sommer. 1990. The “megaprimer” method of site-directed mutagenesis. BioTechniques 8:404-407. [PubMed] [Google Scholar]
- 23.Smith, R. L., and M. E. Maguire. 1998. Microbial magnesium transport: unusual transporters searching for identity. Mol. Microbiol. 28:217-226. [DOI] [PubMed] [Google Scholar]
- 24.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. Dougherty, A. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, J. C. Venter, et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 25.van Vliet, A. H. M., S. Bereswill, and J. G. Kusters. 2001. Ion metabolism and transport, p. 193-206. In H. L. Mobley, G. L. Mendz, and S. L. Hazell (ed.), Helicobacter pylori: physiology and genetics. ASM Press, Washington, D.C. [PubMed]
- 26.van Vliet, A. H. M., E. J. Kuipers, B. Waidner, B. J. Davies, N. de Vries, C. W. Penn, C. M. J. E. Vandenbroucke-Grauls, M. Kist, S. Bereswill, and J. G. Kusters. 2001. Nickel-responsive induction of urease expression in Helicobacter pylori is mediated at the transcriptional level. Infect. Immun. 69:4891-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velayudhan, J., N. J. Hughes, A. A. McColm, J. Bagshaw, C. L. Clayton, S. C. Andrews, and D. J. Kelly. 2000. Iron acquisition and virulence in Helicobacter pylori: a major role for FeoB, a high-affinity ferrous iron transporter. Mol. Microbiol. 37:274-286. [DOI] [PubMed] [Google Scholar]
- 28.Villarreal-Ramos, B., J. Manser, R. A. Collins, G. Dougan, S. N. Chatfield, and C. J. Howard. 1998. Immune responses in calves immunised orally or subcutaneously with a live Salmonella typhimurium aro vaccine. Vaccine 16:45-54. [DOI] [PubMed] [Google Scholar]