Abstract
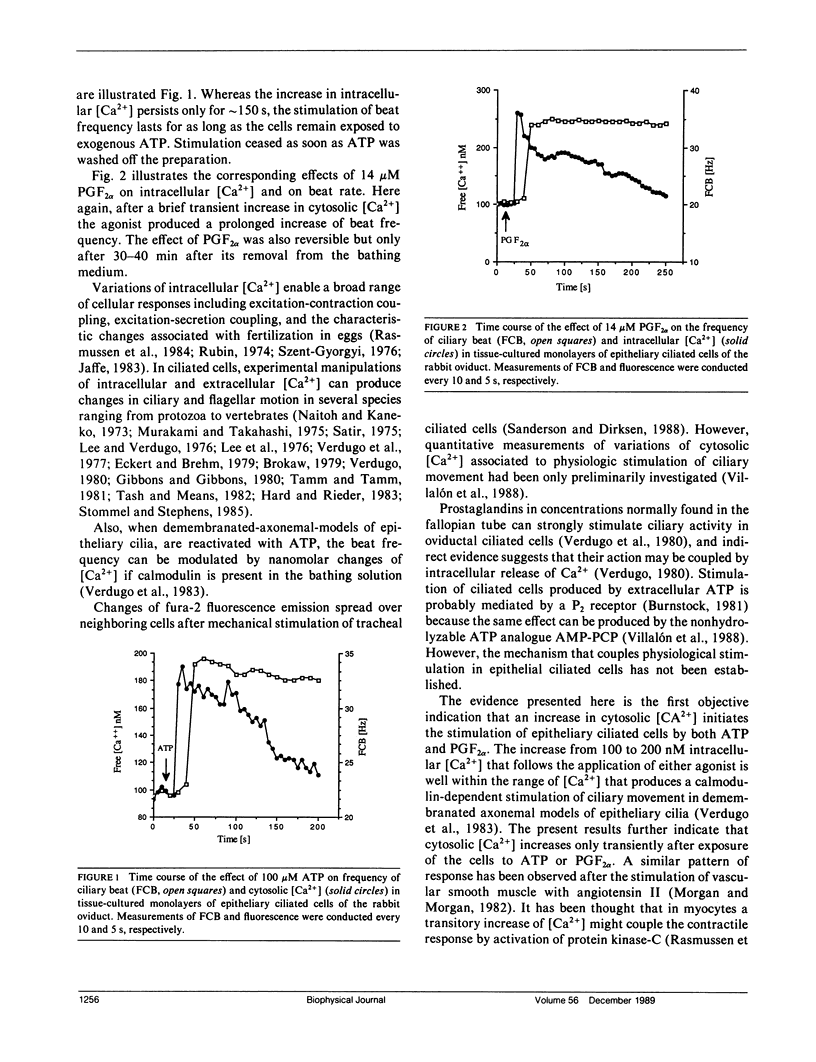
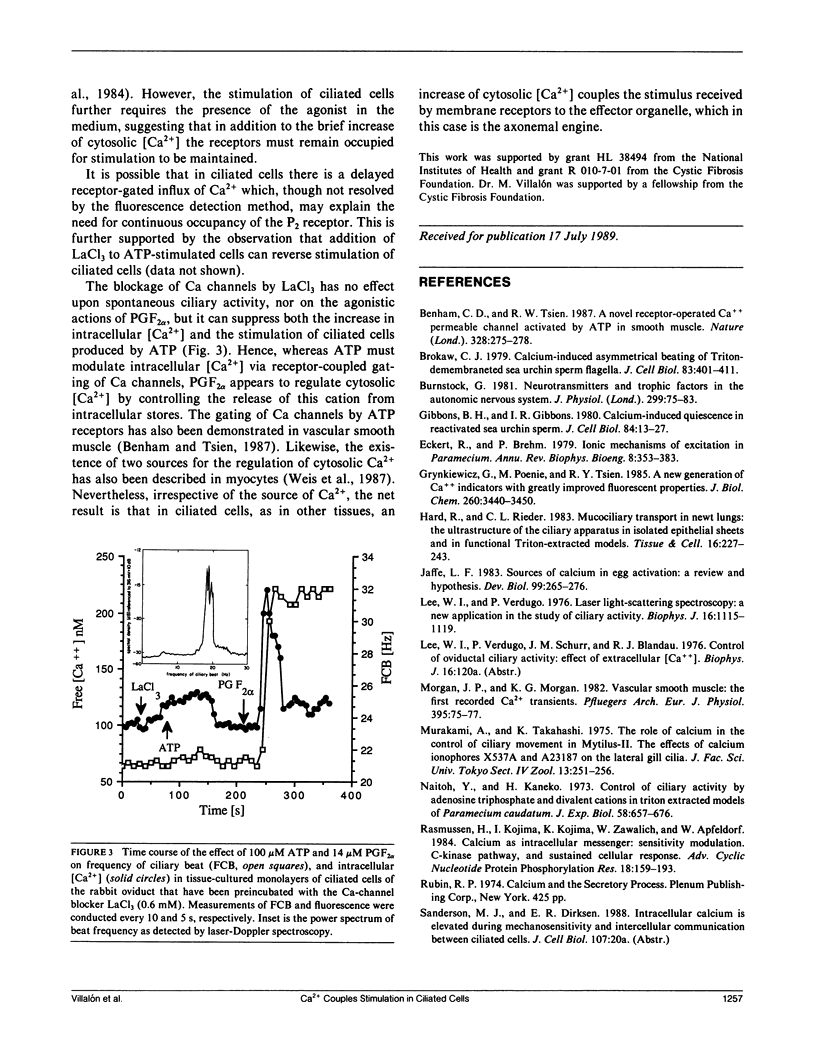
Changes of cytosolic [Ca2+] have been proposed to couple stimulation of ciliary movement, however, quantitative measurements of fluctuations of intracellular free [Ca2+] associated with stimulation of ciliated cells have not been investigated. In primary cultures of rabbit oviductal ciliated cells, the stimulation of ciliary activity produced by micromolar concentrations of adenosine triphosphate (ATP) and prostaglandin F2 alpha (PGF2 alpha) was associated with a transient increase of intracellular [Ca2+]. Whereas the increase of cytosolic [Ca2+] and beat frequency produced by ATP were inhibited by the Ca-channel blocker LaCl3, the rise of cytosolic [Ca2+] and frequency of ciliary beat produced by PGF2 alpha was not affected by LaCl3. These results are the first direct demonstration that fluctuations of cytosolic [Ca2+] are associated with increased ciliary beat frequency in mammalian epithelial cells. The present findings suggest two different calcium-dependent mechanisms for stimulus-coupling in ciliary epithelium: ATP acting via purinergic receptor coupled to transmembrane influx of Ca2+, and PGF2 alpha acting via receptor-mediated release of intracellular sequestered Ca.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Calcium-induced asymmetrical beating of triton-demembranated sea urchin sperm flagella. J Cell Biol. 1979 Aug;82(2):401–411. doi: 10.1083/jcb.82.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Brehm P. Ionic mechanisms of excitation in Paramecium. Annu Rev Biophys Bioeng. 1979;8:353–383. doi: 10.1146/annurev.bb.08.060179.002033. [DOI] [PubMed] [Google Scholar]
- Gibbons B. H., Gibbons I. R. Calcium-induced quiescence in reactivated sea urchin sperm. J Cell Biol. 1980 Jan;84(1):13–27. doi: 10.1083/jcb.84.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hard R., Rieder C. L. Muciliary transport in newt lungs: the ultrastructure of the ciliary apparatus in isolated epithelial sheets and in functional triton-extracted models. Tissue Cell. 1983;15(2):227–243. doi: 10.1016/0040-8166(83)90019-8. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F. Sources of calcium in egg activation: a review and hypothesis. Dev Biol. 1983 Oct;99(2):265–276. doi: 10.1016/0012-1606(83)90276-2. [DOI] [PubMed] [Google Scholar]
- Lee W. I., Verdugo P. Laser light-scattering spectroscopy: a new application in the study of ciliary activity. Biophys J. 1976 Sep;16(9):1115–1119. doi: 10.1016/S0006-3495(76)85760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Vascular smooth muscle: the first recorded Ca2+ transients. Pflugers Arch. 1982 Oct;395(1):75–77. doi: 10.1007/BF00584972. [DOI] [PubMed] [Google Scholar]
- Naito Y., Kaneko H. Control of ciliary activities by adenosinetriphosphate and divalent cations in triton-extracted models of Paramecium caudatum. J Exp Biol. 1973 Jun;58(3):657–676. doi: 10.1242/jeb.58.3.657. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Kojima I., Kojima K., Zawalich W., Apfeldorf W. Calcium as intracellular messenger: sensitivity modulation, C-kinase pathway, and sustained cellular response. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:159–193. [PubMed] [Google Scholar]
- Satir P. Ionophore-mediated calcium entry induces mussel gill ciliary arrest. Science. 1975 Nov 7;190(4214):586–588. doi: 10.1126/science.1103290. [DOI] [PubMed] [Google Scholar]
- Stommel E. W., Stephens R. E. Cyclic AMP and calcium in the differential control of Mytilus gill cilia. J Comp Physiol A. 1985 Oct;157(4):451–459. doi: 10.1007/BF00615145. [DOI] [PubMed] [Google Scholar]
- Tamm S. L., Tamm S. Ciliary reversal without rotation of axonemal structures in ctenophore comb plates. J Cell Biol. 1981 Jun;89(3):495–509. doi: 10.1083/jcb.89.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tash J. S., Means A. R. Regulation of protein phosphorylation and motility of sperm by cyclic adenosine monophosphate and calcium. Biol Reprod. 1982 May;26(4):745–763. doi: 10.1095/biolreprod26.4.745. [DOI] [PubMed] [Google Scholar]
- Verdugo P. Ca2+-dependent hormonal stimulation of ciliary activity. Nature. 1980 Feb 21;283(5749):764–765. doi: 10.1038/283764a0. [DOI] [PubMed] [Google Scholar]
- Verdugo P., Golborne C. E. Remote detection of ciliary movement by fiber optic laser-Doppler spectroscopy. IEEE Trans Biomed Eng. 1988 May;35(5):303–307. doi: 10.1109/10.1385. [DOI] [PubMed] [Google Scholar]
- Verdugo P., Johnson N. T., Tam P. Y. beta-Adrenergic stimulation of respiratory ciliary activity. J Appl Physiol Respir Environ Exerc Physiol. 1980 May;48(5):868–871. doi: 10.1152/jappl.1980.48.5.868. [DOI] [PubMed] [Google Scholar]
- Verdugo P., Raess B. V., Villalon M. The role of calmodulin in the regulation of ciliary movement in mammalian epithelial cilia. J Submicrosc Cytol. 1983 Jan;15(1):95–96. [PubMed] [Google Scholar]
- Verdugo P., Rumery R. E., Tam P. Y. Hormonal control of oviductal ciliary activity: effect of prostaglandins. Fertil Steril. 1980 Feb;33(2):193–196. doi: 10.1016/s0015-0282(16)44541-3. [DOI] [PubMed] [Google Scholar]
- Villalón M., Verdugo P. Hormonal regulation of ciliary function in the oviduct: the effect of beta-adrenergic agonists. Prog Clin Biol Res. 1982;80:59–65. doi: 10.1002/cm.970020713. [DOI] [PubMed] [Google Scholar]
- Weiss G. B., Karaki H., Murakami K. Differentiation of mechanisms for mobilization of calcium in smooth muscle. Can J Physiol Pharmacol. 1987 Apr;65(4):724–728. doi: 10.1139/y87-119. [DOI] [PubMed] [Google Scholar]



