Abstract
We constructed the expression vector pSK-SCP containing the streptococcal exotoxin B gene (spe b) which expressed protease activity. We showed that the recombinant streptococcal pyogenic exotoxin B/streptococcal cysteine protease (rSPE B/SCP) was secreted into the culture supernatant of the transformant and retained its SCP activity, which was equivalent to or greater than that of the naturally occurring molecule. The secreted rSPE B/SCP induced histamine release and degranulation of the human mast cell line HMC-1. This study may contribute to the understanding of the pathogenic role of SPE B/SCP in streptococcal infection and streptococcal toxic shock syndrome.
Streptococcal pyrogenic exotoxin B (SPE B), known as streptococcal cysteine protease (SPE B/SCP) is highly conserved among group A Streptococcus (GAS). The structural gene, spe b, is found among all GAS clinical isolates (20) and expressed in almost all strains (12). This protein is initially secreted as a 42-kDa precursor, zymogen, and is autocatalytically cleaved to a 28-kDa mature SPE B/SCP (7, 9). SPE B/SCP cleaves or degrades fibronectin and vitronectin (12), which function as human matrix proteins. It also cleaves plasma kininogen and releases kinin (10) and cleaves the interleukin-1β (IL-1β) precursor to the active form of IL-1β (12). Furthermore, SPE B/SCP activates the human matrix metalloprotease that increases type IV collagen degradation (2). Finally, genetically inactivated SPE B/SCP is attenuated in the mouse lethality model (15, 16). These findings suggest that SPE B/SCP activates inflammatory responses in the host and plays a very important role in pathogenesis of infections.
The SPE B/SCP gene was previously cloned, sequenced (1, 9, 18), and expressed in Escherichia coli (6, 8, 17, 19). However, the secretion of the active form of the protease from E. coli and its activity relative to the native molecule have not been examined. In order to clarify this question, we constructed an expression vector including spe b and its predicted promoter regions for transformation into E. coli. As a result, recombinant SPE B/SCP (rSPE B/SCP), retaining cysteine protease activity, was obtained from the culture supernatant.
GAS strain NZ131 (kindly provided by D. R. Martin, New Zealand Communicable Disease Center, Porirua) chromosomal DNA was used as the template. Two oligonucleotides (SPEBF0008, 5′GTGTCAACTAACCGTGTTATTG-3′; SPEBR1485, 5′-TGATCTGTGTCTGASTGGATACTT-3′) were designed based on the spe b sequence as reported by Hauser et al. (9) and used as primers. PCR was performed with 25 cycles (94°C for 30 s, 54°C for 30 s, and 75°C for 1.5 min) and pyrobest DNA polymerase (TaKaRa Biomedicals, Kyoto, Japan). In order to construct the expression vector pSK-SCP, a 1,469-bp fragment including spe b and its predicted promoter region was amplified and cloned via the SmaI site in pBluescript II SK(+) plasmid vector. pSK-SCP was transformed into E. coli strain JM109. The rSPE B/SCP was induced with 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Wako Pure Chemical Co., Osaka, Japan) at 37°C with shaking at 175 rpm. After IPTG induction, SCP activity was detected by azocasein assays (14), and it was observed in the culture supernatant but not in the cell lysate. Western blot analysis revealed that recombinant zymogen (rZym) was also secreted. It was suggested that rSPE B/SCP was initially secreted into its culture supernatant as rZym and then cleaved to the mature form autocatalytically.
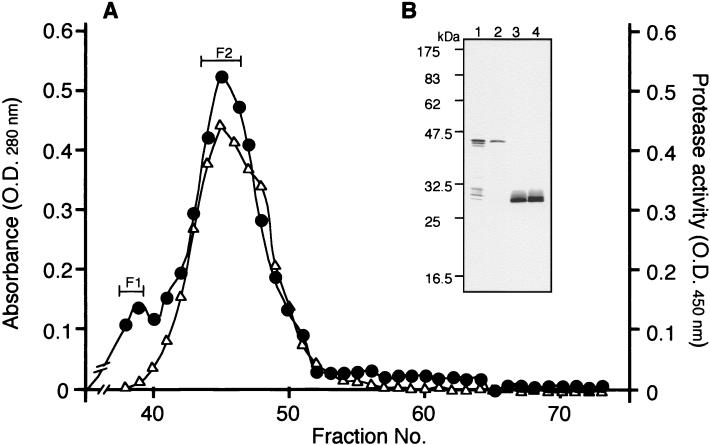
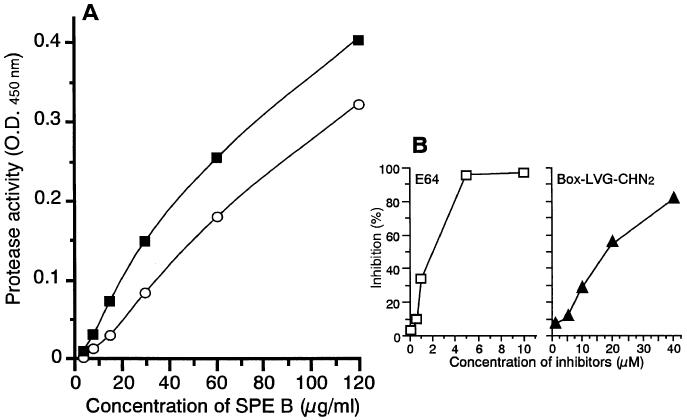
In order to prove this, rSPE B/SCP was purified from culture supernatant by the methods of Kuo et al. (14) and Ohara-Nemoto et al. (18) with some modifications. All purification steps were performed at 4°C. Cysteine protease activity was measured at each step, and fractions expressing the SCP activity were pooled for further preparation. After addition of 1 mM phenylmethylsulfonyl fluoride (Wako Pure Chemical Co.), the supernatant was concentrated by 80% saturated ammonium sulfate precipitation overnight. The pellet was collected and dialyzed against 20 mM Tris-HCl buffer (pH 7.8). The concentrated solution was initially exposed to DEAE-Sepharose CL-6B (Pharmacia Biotech, Uppsala, Sweden) for 30 min, and the unbound sample fraction was separated by the dye resin, matrix Gel Red A (Amicon Co., Lexington, Mass.). The sample equilibrated with 20% ethanol containing 20 mM Tris-HCl buffer (pH 7.0) was applied to the column and eluted by the same buffer after the addition of 2 M NaCl. At the final step, Sephadex G-50 (Pharmacia Biotech) gel filtration was performed to remove contaminating proteins. The final concentration of purified rSPE B/SCP without rZym was 6 to 9 mg/liter of culture volume. The N-terminal amino acid sequences of purified rZym (Fig. 1A, F1) and rSPE B/SCP (Fig. 1A, F2) were analyzed with an automated gas phase amino acid sequencer (Shimazu Corporation, Kyoto, Japan). Both of these sequences matched well the native zymogen (nZym) and native SPE B (nSPE B) sequences, which have been previously described (9). The SCP activities showed a good correlation with the elution pattern of rSPE B/SCP (Fig. 1A, F2). In Western blot analysis with anti-SPE B/SCP antibody, rZym (Fig. 1A, F1) demonstrated a band that corresponded to a molecular mass of approximately 42 kDa, and rSPE B/SCP showed a major band at 28 kDa (Fig. 1B). To compare the SCP activities of nSPE B/SCP and rSPE B/SCP, azocasein assay was performed (14). SCP activity of rSPE B/SCP was the same as or higher than that of nSPE B/SCP (Fig. 2A), and both were inhibited by cysteine protease-specific inhibitors, E64 (Sigma Chemicals Co.) and Box-LVG-CHN2 (Enzyme System Products, Livermore, Calif.) (Fig. 2B), in a dose-dependent manner. These findings were also supported by the results of a skim milk agar plate assay (11) (data not shown).
FIG. 1.
Elution profile and SCP activity of the fraction on Sephadex G-50 and patterns of Western blot analysis. Fractions were monitored for absorbance at 280 nm (closed circle), and the SCP activity was measured by azocasein assay (open triangle). Two peaks (F1 and F2) were detected, and the SCP activity was correlated with the pattern of peak F2. The gas phase amino acid sequence revealed that these two peaks were rZym and rSPE B/SCP. (B) In Western blot analysis, the native or recombinant Zym and SPE B/SCP were incubated with rabbit anti-SPE B/SCP antibody. Lane 1, nZym; lane 2, rZym (peak F1); lane 3, nSPE B/SCP; lane 4, rSPE B/SCP (peak F2).
FIG. 2.
Activities of nSPE B/SCP and rSPE B/SCP. The cysteine protease activity that originated from SPE B/SCP was detected as caseinolytic activity. (A) The SCP activity of rSPE B/SCP (solid squares) was the same as or higher than that of nSPE B/SCP (open circles). (B) The cysteine protease-specific inhibitors E64 (open squares) and Box-LVG-CHN2 (solid triangles) inhibited rSPE B/SCP activity.
Histamine is one of the chemical mediators that participates in the inflammatory process through its capacity of increasing vascular permeability, and/or in the anaphylactic shock. On the other hand, SPE B/SCP is also an inducer of inflammation. We therefore hypothesized that SPE B/SCP may be a pathogenic factor in streptococcal infection of skin and mucous membrane and in streptococcal toxic shock syndrome (STTS) through a histamine releasing activity. However, there is no report examining this relationship in the literature.
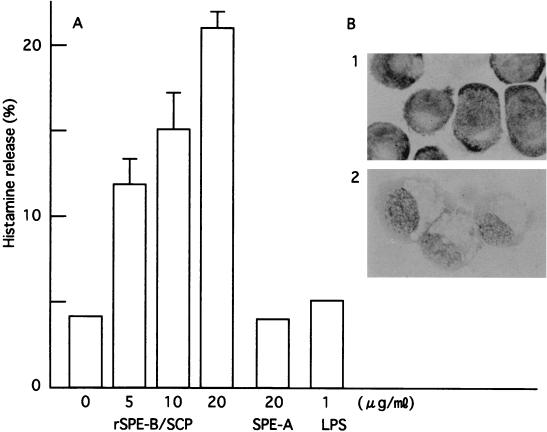
Human leukemic mast cells (3) (HMC-1, kindly provided by Butterfield, Rochester, Minn.) were cultured in Iscove's modified DMEM (Sigma Chemical Co.) supplemented with 10% fetal calf serum (ICN Biomedicals, Inc., Aurora, Ohio), 1.2 mM monothioglycerol (Sigma Chemical Co.), 2 mM glutamine, and antibiotics (streptomycin and penicillin). After washing with Tyrode's solution, 2 × 106 cells/ml were stimulated with rSPE B/SCP, SPE A (Toxin Technology, Inc., Sarasota, Fla.), or lipopolysaccharide (LPS) (Sigma Chemical Co.). After stimulation, histamine in the supernatant was measured in duplicate using a histamine-enzyme-linked immunosorbent assay kit (Immunotech a Beckman Coulter Co., Marseille, France) according to the manufacturer's instructions. The degranulations from HMC-1 stimulated with rSPE B/SCP were observed morphologically after 0.05% toluidine blue (pH 5.0) staining. As shown in Fig. 3A, histamine was released from HMC-1 cells stimulated with SPE B/SCP in a dose-dependent manner. SPE A and LPS, however, did not induce histamine release from HMC-1 cells. Histamine release from HMC-1 cells was observed at 2 min after stimulation with SPE B/SCP, reaching a maximum at 20 min. The histamine-releasing capacity of rSPE B/SCP was abolished by heating at 60°C for 30 min, and it was inhibited by E64 (data not shown). It is suggested that the histamine-releasing capacity may be correlated with the enzymatic activity of SPE B/SCP. The release of granules from HMC-1 stimulated with rSPE B/SCP was observed morphologically (Fig. 3B). Histamine released by SPE B/SCP may play a role in STTS and/or streptococcal infection of skin and mucous membrane.
FIG. 3.
Histamine release and degranulation from HMC-1 cells stimulated with rSPE B/SCP. HMC-1 cells were incubated at 2 × 106 cells/ml in Tyrode's solution for 20 min at 37°C with rSPE B/SCP, SPE A, and LPS. After incubation, histamine in the supernatant was measured by enzyme-linked immunosorbent assay. (A) Data are expressed as a percentage of release of histamine in comparison to control. As a control, the HMC-1 cells were allowed to freeze and thaw for three cycles to release the remaining histamine from the cells. (B) Degranulation pattern from HMC-1 cells stimulated with rSPE B/SCP. Panel 1, control (nonstimulated HMC-1 cells); panel 2, rSPE B/SCP-stimulated HMC-1 cells.
Generally, recombinant proteins that were expressed in E. coli without any modifications were overexpressed in cytoplasm as inclusion body. To minimize inclusion body formation, regulation of expression of the proteins is required. spe b was ligated into pBluescript II SK(+) with its deduced promoter sequences (−35, −10, and Shine-Dalgarno sequences, respectively). We assumed that E. coli would recognize these promoter sequences and express rSPE B/SCP using these regions. The first four residues of signal sequences of SPE B/SCP, MNKK, were very similar to the secretion signal of E. coli (MK). Consequently, rZym secreted into the culture supernatant fluids could be converted to its active form following proteolysis and reduction.
It was suggested that SPE B production is regulated by Rgg protein in trans (4). Recently, Chaussee et al. (5) showed that some extracellular proteins, including SPE B, are positively regulated by Rgg at the transcription level. Although pSK-SCP did not include rgg, which encodes Rgg, rSPE B/SCP could be expressed. It is possible that the inserted promoter regions functioned as the regulator in cis to influence rSPE B/SCP production.
In conclusion, rSPE B/SCP was expressed in E. coli as a secretion protein that demonstrated high SCP activity. rSPE B/SCP was able to release histamine from HMC-1 cells. We have found that the plasma histamine levels in four of the seven patients with STTS were higher than those in healthy subjects and that the injection of the rSPE B/SCP in the skin of guinea pigs increased the capillary permeability (submitted for publication). Purified rSPE B/SCP, having the same enzymatic activity as the naturally produced molecule, may provide a reagent free of other streptococcal products to examine the role of SPE B/SCP in host-pathogen interaction in GAS infections.
Nucleotide sequence accession number.
The sequence of spe b from GAS, strain NZ131, has been deposited in the DDBJ database under accession no. AB0512398.
Acknowledgments
This work was supported by a grant from Research on Emerging and Reemerging Infectious Diseases, Health Sciences Research Grant, the Ministry of Health, Labor, and Welfare of Japan.
Editor: J. T. Barbieri
REFERENCES
- 1.Bohach, G. A., A. R. Hauser, and P. M. Schlievert. 1988. Cloning of the gene, speB, for streptococcal pyrogenic exotoxin type B in Escherichia coli. Infect. Immun. 56:1665-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns, E. H., A. M. Merciel, and J. M. Musser. 1996. Activation of a 66-kilodalton human matrix metalloproteinase by Streptococcus pyogenes extracellular cysteine protease. Infect. Immun. 64:4744-4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butterfield, J. H., D. Weiler, G. Dewald, and G. J. Gleich. 1988. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leukoc. Res. 12:345-355. [DOI] [PubMed] [Google Scholar]
- 4.Chaussee, M. S., D. Ajdic, and J. J. Ferretti. 1999. The rgg gene of Streptococcus pyogenes NZ131 positively influences extracellular SPE B production. Infect. Immun. 67:1715-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaussee, M. S., R. O. Watson, J. C. Smoot, and J. M. Musser. 2001. Identification of Rgg-regulated exoproteins of Streptococcus pyogenes. Infect. Immun. 69:822-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doran, J. D., M. Nomizu, S. Takebe, R. Menard, D. Griffith, and E. Zimek. 1999. Autocatalytic processing of streptococcal cysteine protease zymogen. Processing mechanism and characterization of the autoproteolytic cleavage sites. Eur. J. Biochem. 263:145-151. [DOI] [PubMed] [Google Scholar]
- 7.Gerlach, D., H. Knoll, W. Kohler, J. H. Ozegowski, and V. Hribalova. 1983. Isolation and characterization of erythrogenic toxins. V. Communications: identity of erythrogenic exotoxin type B and streptococcal proteinase precursor. Zentbl. Bakteriol. Mikrobiol. Hyg. Int. Abt. Orig. A 255:221-223. [PubMed] [Google Scholar]
- 8.Gubba, S., D. E. Low, and J. M. Musser. 1998. Expression and characterization of group A Streptococcus extracellular cysteine protease recombinant mutant proteins and documentation of seroconversion during human invasive disease episodes. Infect. Immun. 66:765-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauser, A. R., and P. M. Schlievert. 1990. Nucleotide sequence of streptococcal pyogenic exotoxin type B gene and relationship between the toxin and the streptococcal proteinase precursor. J. Bacteriol. 172:4536-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herwald, H., M. Collin, W. Muller-Esterl, and L. Bjorck. 1996. Streptococcal cysteine protease releases kinins: a novel virulence mechanism. J. Exp. Med. 184:665-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hynes, W. L., and J. R. Tagg. 1985. A simple plate assay for detection of group A Streptococcus proteinase. J. Microbiol. Methods 4:25-31. [Google Scholar]
- 12.Kapur, V., S. Topouzis, M. W. Majesky, L.-L. Li, M. R. Hamrick, R. J. Hamill, J. M. Patti, and J. M. Musser. 1993. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degradates vitronectin. Microb. Pathog. 15:327-346. [DOI] [PubMed] [Google Scholar]
- 13.Kapur, V., M. W. Majeski, L.-L. Li, R. A. Black, and J. M. Musser. 1993. Cleavage of interleukin 1β (IL-1β) precursor to produce active IL-1β by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 90:7676-7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo, C.-F., J.-J. Wu, K.-Y. Lin, P.-J. Tsai, S.-C. Lee, Y.-T. Jin, H.-Y. Lei, and Y.-S. Lin. 1998. Role of streptococcal pyogenic exotoxin B in the mouse model of group A streptococcal infection. Infect. Immun. 66:3931-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukomski, S. S., S. Sreevatsan, C. Amberg, W. Reichardt, M. Woischnik, A. Podbielski, and J. M. Musser. 1997. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decrease mouse lethality of serotype M3 and M49 strain s. J. Clin. Investig. 99:2574-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukomski, S., E. H. Burns, Jr., P. R. Wyde, A. Podbielski, J. Rurandgirwa, D. K. Moore-Poveda, and J. M. Musser. 1998. Genetic inactivation of an extracellular cysteine protease (Spe B) expressed by Streptococcus pyogenes decrease resistance to phagocytosis and dissemination to organs. Infect. Immun. 66:771-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musser, J. M., K. Stockbauer, V. Kapur, and G. W. Rudgers. 1996. Substitution of cysteine 192 in a highly conserved Streptococcus pyogenes extracellular cysteine protease (interleukin 1β convertase) alters proteolytic activity and ablates zymogen processing. Infect. Immun. 64:1913-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohhara-Nemoto, Y., M. Sasaki, and M. Kaneko. 1994. Cysteine protease activity of streptococcal pyrogenic exotoxin B. Can. J. Microbiol. 40:930-936. [DOI] [PubMed] [Google Scholar]
- 19.Toyosaki, T., T. Yoshida, Y. Tsuruta, T. Yutsudo, M. Iwasaki, and R. Suzuki. Definition of the mitogenic factor (MF) as a novel streptococcal superantigen that is different from streptococcal pyrogenic exotoxins A, B, and C. Eur. J. Immunol. 26:2639-2701. [DOI] [PubMed]
- 20.Yu, C. E., and J. J. Ferretti. 1991. Frequency of the erythrogenic toxin B and C genes (speB and speC) among clinical isolates of group A streptococci. Infect. Immun. 59:211-215. [DOI] [PMC free article] [PubMed] [Google Scholar]