Abstract
The vaccine efficacy of the gene sequence encoding the signal peptide of the antigen known as antigen 2 or proline-rich antigen (Ag2/PRA), an immunodominant antigen present in the cell wall of the fungal pathogen Coccidioides immitis, was investigated in a murine model of coccidioidomycosis. Expression plasmids for Ag2/PRA(1-18) DNA (signal sequence), Ag2/PRA(19-194) DNA (lacking the signal sequence), and Ag2/PRA(1-194) DNA (full length) were inserted in the pVR1012 vector, and the constructs were used to vaccinate the highly susceptible BALB/c mouse strain. Immunization with the signal gene sequence significantly reduced the fungal burden in the lungs and spleens of mice 12 days after intraperitoneal challenge with a lethal dose of 2,500 C. immitis arthroconidia, to a level comparable to the protection induced in mice immunized with the full-length Ag2/PRA(1-194) DNA. The Ag2/PRA(19-194) gene protected mice but to a significantly lower level than the signal sequence or the full-length Ag2 gene. The immunizing capacity of Ag2/PRA(1-18) was not attributable to a nonspecific immunostimulatory effect of DNA, as evidenced by the fact that mice immunized with a frameshift mutation of Ag2/PRA(1-18) were not protected against challenge. Furthermore, a synthetic peptide corresponding to the translated sequence of Ag2/PRA(1-18) DNA protected mice, albeit at a lower level than the Ag2/PRA(1-18) DNA vaccine. The protection induced with the signal gene vaccine correlated with the production of gamma interferon when splenocytes from Ag2/PRA(1-18)-immunized mice were stimulated with recombinant full-length Ag2 and was not associated with the production of anti-Coccidioides immunoglobulin G antibody. This is the first study to establish that a signal peptide sequence alone, administered as a gene vaccine or synthetic peptide, can induce protective immunity against a microbial pathogen.
Coccidioidomycosis (San Joaquin Valley fever) is a respiratory disease caused by the primary fungal pathogen Coccidioides immitis (27, 33). The incidence of coccidioidomycosis in the southwestern United States sharply increased in the early 1990s as a result of environmental and demographic changes (28). Primary infection occurs via inhalation of mycelial-phase arthroconidia, which enter the alveoli and undergo a morphogenetic conversion to a parasitic, spherule form. The disease has diverse manifestations, ranging from a benign pulmonary infection to a progressive disseminated disease most commonly involving the skin, bones, and/or joints and central nervous system. Evidence from clinical and experimental investigations revealed that the severity of coccidioidomycosis directly correlates with depressed cell-mediated immunity to coccidioidal antigens (2, 4, 7-10, 25). Recovery from primary infection is associated with strong cell-mediated immune responses to C. immitis and is accompanied by life-long immunity to exogenous reinfection (6, 33). Therefore, developing a vaccine is a feasible and promising strategy against C. immitis in regions where this fungal pathogen is endemic.
Previous vaccine studies using experimental animal models demonstrated that immunization with killed spherules induces protection against pulmonary challenge with a lethal dose of arthroconidia (20, 22, 23). Investigations showed that the protective component(s) resided primarily in the cell walls of killed spherules (20, 22, 29) and that cell wall extracts enriched in antigen 2 (Ag2) induced protection against challenge with C. immitis (5, 21). To directly evaluate the vaccine efficacy of Ag2, the gene that encodes this antigen was cloned (35, 36) and the protective capacity of the Ag2 DNA vaccine was compared with that of the recombinant Ag2 protein vaccine in a murine model of coccidioidomycosis (15). Mice immunized with the Ag2 DNA vaccine showed a significant decrease in the fungal burden in their lungs and spleens after intraperitoneal (i.p.) challenge with 2,500 arthroconidia of C. immitis. Recombinant Ag2, expressed in Escherichia coli as a glutathione S-transferase (GST) fusion peptide, showed a reduced efficacy in inducing protective immunity compared with the gene vaccine. In related studies, Dugger et al. (13) cloned a gene that encoded a proline-rich antigen (PRA) and in subsequent studies, recombinant PRA and the PRA gene were reported to protect BALB/c mice against i.p. challenge with 50 arthroconidia (1, 19). The gene that encodes Ag2 has sequence identity with the gene that encodes PRA (13, 35), hence the designation Ag2/PRA.
In preliminary analyses of Ag2/PRA-derived cDNA constructs, we obtained evidence that an Ag2/PRA construct which lacked the N-terminal signal sequence was less protective than the full-length Ag2/PRA(1-194) sequence. This finding, coupled with reports by others that DNA vaccines without the leading signal sequence are less immunogenic than their full-length counterparts (3, 12, 14, 18), suggested that the signal sequence may be important in inducing protective immunity. To explore this possibility, we created a construct containing the coding region for the Ag2/PRA(1-18) signal peptide domain of Ag2/PRA and evaluated its vaccine efficacy in comparison with that of a full-length DNA vaccine [Ag2/PRA(1-194)] and a DNA with the signal sequence removed [Ag2/PRA(19-194)].
MATERIALS AND METHODS
Vaccine plasmid construction.
The full-length Ag2/PRA cDNA, which consists of a 582-bp open reading frame and encodes a 194-amino-acid protein, was subcloned into the XbaI-and-BamHI site of the eukaryotic expression vector plasmid pVR1012 (Vical, Inc., San Diego, Calif.) as previously reported (15).
The pVR1012-Ag2/PRA(1-18) DNA was constructed by the following procedure. First, the DNA sequence encoding amino acids 1 to 18 of the N terminus was amplified on the template plasmid pVR1012-Ag2/PRA(1-194) by PCR with upstream (5′-TTGGGATCCGTCGACATGCAGTTCTCTCAC-3′) and downstream (5′-GGAAGATCTCGAGTTAGGCACTGGCGAGGCC-3′) primers. The reaction conditions were 95°C for 4 min, 30 cycles of 95°C for 60 s, 60°C for 30 s, and 72°C for 45 s, and then a final extension at 72°C for 7 min. The PCR fragment was digested with BamHI and BglII, purified on a 3% agarose gel, and then ligated into pVR1012 vector (Vical, Inc.). After transformation, a positive colony was picked from Luria broth agar containing kanamycin and sequenced for confirmation of signal peptide sequence in the correct reading frame. A similar strategy was used to generate the recombinant pVR1012-Ag2/PRA(19-194) DNA construct (34). Where indicated, the Ag2/PRA(19-194) construct was also ligated to the pVR1020 plasmid (Vical, Inc.) which contains the tissue plasminogen activator (TPA) signal sequence (11, 24).
Preparation of plasmid DNA.
To prepare plasmid DNA for immunization, E. coli DH5α cells were transformed with the DNA constructs or the vector plasmid alone and then cultured at 37°C for 16 h in Luria broth supplemented with kanamycin (50 μg/ml). Plasmid DNA was isolated by using an EndoFree plasmid purification kit (Qiagen, Santa Clara, Calif.). Plasmid DNA was resuspended in USP saline (Baxter Healthcare Corporation, Deerfield, Ill.) and stored at −20°C until used. The endotoxin level was less than 0.1 U/μg of DNA for each construct, as assayed with the QCL-1000 Limulus amebocyte lysate kit (BioWhittaker, Walkersville, Md.).
Gene expression of plasmid DNA in mammalian cells.
To establish that the pVR1012-based Ag2/PRA constructs would be expressed in mammalian cells, a transient-transfection assay was performed (16). Using the Lipofectamine Plus kit (GIBCO, Grand Island, N.Y.), 2 μg each of Ag2/PRA(1-194) DNA, Ag2/PRA(19-194) DNA, and Ag2/PRA(1-18) DNA was transfected into nontumorigenic CL-7 fibroblast cells which were originally derived from BALB/c mice (31). The cells were grown in Dulbecco's minimal essential medium (GIBCO) supplemented with 10% fetal calf serum and 4.5 mg of glucose per ml and then plated into six-well plates at a concentration of 4 × 105 cells/well. After overnight incubation, the cells were transfected and, 3 days later, were harvested, and total RNA was isolated for reverse transcription (RT)-PCR. The RNA samples from the transfected cells were analyzed simultaneously with the primers for each Ag2/PRA construct.
The RNA was transcribed into cDNA by using reverse transcriptase (Perkin-Elmer Inc., Boston, Mass.). The primers for Ag2/PRA(1-18) were those mentioned above. The following primers were used for mRNA expression of Ag2/PRA(1-194) and Ag2/PRA(19-194): upstream, 5′-ATGCAGTTCTCTCACGCTCTCATC-3′ and 5-′ATGCAGCTCCCAGACATCCCACCT-3′, respectively, and downstream, 5′-TTACAGGTAGGCAGCGAGACC-3′ for both. The conditions for the PCR were as previously reported (16). All PCR products were electrophoresed in 2.5% agarose gel to confirm the size of fragments.
Synthesis of Ag2/PRA signal peptide.
The 18-amino-acid peptide (MQFSHALIALVAAGLASA) corresponding to the signal sequence of Ag2/PRA was synthesized as a chloride salt by Bachem Bioscience (King of Prussia, Pa.) and the Protein Core Facility at the University of Texas Health Science Center, San Antonio, using 9-fluorenylmethoxy carbonyl-solid phase synthesis and high-pressure liquid chromatography purification techniques. Electrospray mass spectrophotometry and amino acid analysis were used to verify that the Ag2/PRA(1-18) peptide preparations had the correct molecular weight and predicted amino acid content. Lyophilized peptides were stored under nitrogen gas before reconstitution in sterile 100% dimethyl sulfoxide (DMSO) to a concentration of 2 mg/ml.
Immunization.
Five-week-old female BALB/c (H-2d) mice were purchased from Jackson Laboratory (Bar Harbor, Maine). The mice were maintained for at least 1 week before use.
DNA immunization was performed by injecting groups of 10 mice intramuscularly with 50 μg of either Ag2/PRA(1-194) DNA, Ag2/PRA(19-194) DNA, Ag2/PRA(1-18) DNA, or the pVR1012 vector alone, each in 50 μl of physiologic saline. Before each injection, the mice were lightly anesthetized via inhalation of methoxyflurane (Metofane; Mallinckrodt Veterinary, Inc., Mundelein, Ill.). Injections were given in the tibialis anterior muscle in a site which had been pretreated with Nair (Carter-Wallace, Inc., New York, N.Y.) 1 day before the first immunization. A total of three immunizations were given at weekly intervals in alternating sites on the left and right hind legs. Mice were challenged 2 weeks after the final immunization.
Immunization with the Ag2/PRA(1-18) synthetic peptide was performed by injecting mice intramuscularly with 100 μl containing 10 μg of the peptide diluted in 12.5% DMSO and admixed with an equal volume of complete Freund adjuvant. To minimize peptide precipitation during emulsification, 250 μl of the DMSO-solubilized peptide solution was drawn into a polypropylene syringe (Air-tite Products, Virginia Beach, Va.) containing 1 ml of adjuvant and then mixed with 750 μl of sterile saline using a three-way stopcock (Baxter Healthcare Corporation). Mock-immunized control mice received 100 μg of DMSO-adjuvant alone. Seven and 14 days after the first immunization, the mice were given subcutaneous injections of 100 μl containing 10 μg of the DMSO-solubilized peptide in incomplete Freund adjuvant.
Infection and assessment of disease severity.
Arthroconidia were harvested from 4- to 8-week-old mycelium-phase cultures of C. immitis strain Silveira. The arthroconidium suspensions were passed over a sterile nylon column to remove hyphal elements, and the cells were enumerated by hemacytometer counts. Mice were infected by an i.p. injection with 2,500 arthroconidia suspended in 0.5 ml of pyrogen-free saline or, to simulate the natural route of infection, an intranasal instillation of 10 arthroconidia. The viability of the inocula was confirmed by plate counts on 1% glucose-2% yeast extract agar. All manipulations using viable C. immitis or infected tissues were performed in certified biosafety level 3 facilities.
Vaccine-induced protection was evaluated by quantifying C. immitis CFU in the lungs and spleens 12 days postinfection as detailed previously (15-17). Results were expressed as log10 CFU per organ. In selected experiments, the protective capacity of Ag2/PRA DNA constructs was evaluated in terms of survival of mice at days 1 through 40 postinfection.
IFN-γ induction and analyses.
To evaluate the induction of gamma interferon (IFN-γ), spleens were isolated from mice 12 days after challenge, and single-cell suspensions were prepared by gentle homogenization. Cells were suspended in cold Hank's balanced salt solution and treated with isotonic ammonium chloride to lyse erythrocytes. The splenocytes were washed by centrifugation, resuspended in Dulbecco's minimal essential medium containing 10% fetal bovine serum, and dispensed into wells on a microtiter plate at a concentration of 2 × 106 mononuclear cells per well. The cultures were stimulated with 50 μg of recombinant Ag2/PRA per ml, which was expressed as a GST-Ag2/PRA(1-194) fusion protein (35), or 1 μg of synthetic Ag2/PRA(1-18) peptide per ml. After a 48-h incubation at 37°C under 5% CO2, supernatants were collected and passed through a 0.45-μm filter to remove viable cells. IFN-γ was assayed by a two-site sandwich enzyme-linked immunoassay (ELISA) using affinity-purified rat immunoglobulin G1 (IgG1) anti-mouse antibodies (PharMingen, San Diego, Calif.) as previously reported (15-17).
Analysis of serum IgG isotypes.
Humoral immune responses were assessed by quantifying IgG1 and IgG2a isotype antibodies with an ELISA (17). Serum was collected from mice 12 days after challenge, filtered through a 0.45-μm filter, and stored at −20°C until assayed. Twofold serial dilutions were prepared, and 0.1 ml of the diluted serum specimens was added to wells on a 96-well microtiter plate which had been coated with 100 ng of GST-Ag2(1-194). Goat anti-mouse IgG1 and IgG2a antibodies (1:1,000 dilution), each conjugated to alkaline phosphatase (Zymed, South San Francisco, Calif.), were used as second antibodies. The plates were washed, and the p-nitrophenyl phosphate substrate (Sigma) was added to obtain color development in 30 min. Plates were read at 410 nm on an MR-5000 microplate reader (Dynatech Inc., Lake Success, N.Y.). A serum pool prepared from more than 50 mice immunized with Ag2/PRA was used as a reference standard for quality control.
Statistical analyses.
The data were analyzed with the Wilcoxon rank-sum test. Differences in the survival of mice at days 1 through 40 postinfection were analyzed by the Kaplan-Meier procedure. Probability values of <0.05 were considered significant.
RESULTS
In vitro expression of Ag2/PRA DNA plasmids in BALB/c mouse-derived cells.
The ability of pVR1012-based Ag2/PRA gene constructs to transfect mammalian cells was tested in vitro in the CL-7 cell line. As shown in Fig. 1, a 66-bp band was amplified by PCR using cDNA template isolated from CL-7 cells transfected with Ag2(1-18) DNA. PCR products of 582 and 520 bp were obtained from cDNA samples from cells transfected with Ag2/PRA(1-194) and Ag2/PRA(19-194), respectively. Total RNA from CL-7 cells transfected with pVR1012 vector DNA showed no RT-PCR product. As CL-7 is a cell line established from the BALB/c mouse strain (31), these results demonstrated that the Ag2/PRA DNA vaccine constructs have the potential to be expressed in vivo in BALB/c mice.
FIG. 1.
Expression of Ag2/PRA constructs in the CL-7 cell line as determined by RT-PCR. The pVR1012-based plasmids of Ag2/PRA(1-18), Ag2/PRA(19-194), and Ag2/PRA(1-194) and, as a control, pVR1012 alone were transfected into CL-7 cells. Total RNA was isolated at day 3 after transfection, and Ag2/PRA mRNA expression was assayed by RT-PCR. The PCR products were visualized on a 2% agarose gel.
Protective capacity of Ag2/PRA signal sequence gene vaccine.
Because of our earlier finding that a Ag2/PRA(1-194) DNA vaccine was more effective than recombinant Ag2/PRA protein in inducing protective immunity against C. immitis (15), we utilized DNA-based immunizations to assess the protective capacity of the Ag2/PRA-derived constructs. Preliminary experiments indicated that cDNA constructs lacking the signal sequence were less protective than the full-length cDNA. This observation was borne out by the data presented in Fig. 2. Consistent with an earlier report (15), mice immunized with the Ag2/PRA(1-194) DNA construct ligated to the pVR1012 vector showed a significant reduction in the number of CFU recovered from their lungs (P < 0.0001) and spleens (P < 0.0001) compared to the control group which received the pVR1012 vector alone. Ag2/PRA(19-194) DNA, which lacks the signal peptide sequence, also induced protection against i.p. challenge compared to the vector control mice (P < 0.0001) but was significantly less protective than Ag2/PRA(1-194) DNA (P < 0.0001). Mice immunized with Ag2/PRA(1-18) DNA (signal sequence) were strongly protected, to a level comparable to that observed in mice immunized with the full-length Ag2/PRA DNA.
FIG. 2.
Vaccine efficacy of the full-length Ag2/PRA(1-194) DNA, signal sequence-truncated Ag2/PRA(19-194) DNA, and the signal sequence Ag2/PRA(1-18) DNA in BALB/c mice compared to immunization with the pVR1012 vector alone. The fungal CFU in the lungs and spleens were enumerated 12 days after i.p. challenge with 2,500 arthroconidia. Results are means ± standard errors obtained in four separate experiments, each involving nine or more mice per group.
The preceding results established that immunization of mice with the Ag2/PRA(1-18) signal sequence DNA effected a reduction in the fungal load in lungs and spleens 12 days after i.p. challenge. The protective capacity of the Ag2/PRA(1-18) DNA vaccine was further evaluated by monitoring survival in mice over a 40-day period following an i.p. challenge with 2,500 arthroconidia. As shown in Fig. 3, 60% of mice immunized with Ag2/PRA(1-18) DNA survived, while all mice immunized with the pVR1012 vector alone died within 25 days (P < 0.0001). By comparison, 90% of mice vaccinated with Ag2/PRA(1-194) DNA survived challenge, which was greater than the survival of mice vaccinated with Ag2/PRA(1-18) DNA but not significantly so according to the Kaplan-Meier procedure.
FIG. 3.
Survival of BALB/c mice immunized with Ag2/PRA(1-194) (full-length) DNA, Ag2/PRA(1-18) (signal gene) DNA, or the pVR1012 vector and then challenged with 2,500 arthroconidia via an i.p. route. Each group consisted of 10 mice.
Whereas immunization with Ag2/PRA(1-18) DNA induced protection in mice challenged with 2,500 arthroconidia by the i.p. route, the signal peptide gene vaccine did not protect mice against pulmonary challenge with 10 arthroconidia (data not shown). This is consistent with our previous report that neither the Ag2/PRA(1-194) DNA vaccine nor recombinant GST-Ag2/PRA(1-194) induced protection against pulmonary challenge, as assessed by measuring the fungal load in the lungs 12 days after challenge or survival of the mice within a 30-day period after challenge (15).
To determine if the protective capacity of the Ag2/PRA(1-18) DNA might be attributable to a nonspecific immunopotentiation from nucleotide sequences (32), a frameshift mutation of the Ag2/PRA(1-18) was created with PCR by deleting the first nucleotide in the second codon. Mice immunized with DNA containing the frameshift mutation [designated Ag2/PRA(M1-18)] were not protected against i.p. challenge with 2,500 arthroconidia, whereas mice immunized with the correct Ag2/PRA(1-18) DNA construct showed a >1-log decrease in CFU in their lungs and spleens compared to the pVR1012 vector control mice (Fig. 4).
FIG. 4.
Vaccine efficacy of Ag2/PRA(1-18) DNA and Ag2/PRA(M1-18) DNA, each ligated to the pVR1012 vector, in BALB/c mice challenged by an i.p. route with 2,500 arthroconidia. Results are means ± standard errors in tissues from groups of 10 mice at day 12 postinfection.
Protective efficacy of synthetic Ag2/PRA(1-18) signal peptide.
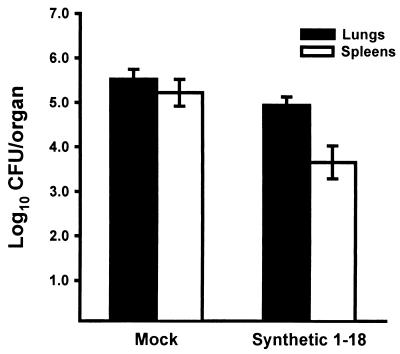
To determine if the vaccine efficacy of the Ag2/PRA(1-18) DNA could be reproduced by using a synthetic Ag2/PRA(1-18) peptide, mice were given three weekly immunizations of 10 μg of a synthetic Ag2/PRA(1-18) peptide which had been dissolved in DMSO (final concentration of 12.5%) and then mixed with Freund adjuvant. Control mice received an identical amount of DMSO alone in adjuvant. The results (Fig. 5) established that the synthetic Ag2/PRA(1-18) peptide induced protection, as measured by reduced fungal load in the spleens (P < 0.008) after an i.p. challenge. In a separate experiment, the protective capacity of the Ag2/PRA(1-18) synthetic peptide was compared directly with that of the Ag2/PRA(1-18) DNA construct. Mice immunized with the synthetic peptide showed a mean lung log10 CFU of 4.9 compared to a mean lung log10 CFU of 2.8 in mice immunized with pVR1012-Ag2/PRA(1-18) DNA, thereby establishing that the synthetic peptide is significantly less protective than the signal sequence DNA (P < 0.005).
FIG. 5.
Vaccine efficacy of synthetic peptide encoded by Ag2/PRA(1-18) DNA in BALB/c mice challenged by an i.p. route with 2,500 arthroconidia. Mice were immunized with the synthetic peptide dissolved in DMSO and admixed with Freund adjuvant. Mock controls were immunized with the same concentration of DMSO in adjuvant. Results are means ± standard errors for groups of 10 mice at day 12 postinfection.
Induction of IFN-γ by Ag2/PRA DNA constructs.
We have previously reported that Ag2/PRA(1-194) cDNA induces the T-helper 1-associated cytokine IFN-γ (15, 17). To determine if the protection induced by the Ag2/PRA(1-18) DNA vaccine was associated with the production of this cytokine, splenocytes were collected from mice 12 days after i.p. challenge and stimulated in vitro with recombinant Ag2/PRA, which contained the full-length sequence with an N terminus GST peptide moiety (35). The results are shown in Fig. 6. Spleen cells from mice immunized with Ag2/PRA(1-194) DNA secreted 1,422 pg of IFN-γ in response to stimulation with recombinant GST-Ag2/PRA(1-194) (Fig. 6A). Splenocytes from mice immunized with Ag2/PRA(19-194) and Ag2/PRA(1-18) secreted 663 and 868 pg/ml, respectively. The IFN-γ responses did not differ significantly between the three groups of mice, and all three groups showed significant IFN-γ production compared to the pVR1012 vector control mice (P < 0.05). These results establish that all three constructs express one or more IFN-γ-inducing epitopes, and on the basis that there are no sequence homologies between the Ag2/PRA(1-18) DNA and Ag2/PRA(19-194) DNA (35, 36), the epitopes expressed by these two constructs differ in composition. This concept was confirmed by the results shown in Fig. 6B, wherein the synthetic Ag2/PRA(1-18) peptide induced IFN-γ production in spleen cells from mice immunized with Ag2/PRA(1-194) DNA or Ag2/PRA(1-18) DNA but not Ag2/PRA(19-194).
FIG. 6.
IFN-γ production by in vitro-stimulated spleen cells from mice immunized with Ag2/PRA(1-194), Ag2/PRA(19-194), or Ag2/PRA(1-18), each ligated to pVR1012. Spleen cells were pooled from groups of 10 mice and stimulated with the full-length GST-Ag2/PRA recombinant protein (A) or the synthetic signal peptide (B) for 48 h, and the supernatants were assayed for IFN-γ by an ELISA.
Humoral immune response to Ag2/PRA cDNA constructs.
To further characterize the immune response to the constructs, serum was collected from immunized, infected mice 12 days after challenge and assayed for anti-Ag2/PRA IgG isotype response using the recombinant GST-Ag2/PRA fusion protein as the target antigen. Consistent with an earlier report (17), mice immunized with Ag2/PRA(1-194) DNA ligated to the pVR1012 vector produced both IgG1 and IgG2a, at an approximate ratio of 5:1 (Fig. 7). The truncated Ag2/PRA(19-194) DNA induced a similar profile of antibody response, although the level of IgG1 was decreased. In marked contrast to the antibody response induced by the full-length Ag2/PRA(19-194) DNA and Ag2/PRA(19-194) DNA, neither anti-Coccidioides IgG1 nor IgG2a was detected in sera of mice immunized with Ag2/PRA(1-18) DNA.
FIG. 7.
IgG1 (A) and IgG2a (B) isotype responses in mice vaccinated with Ag2/PRA(1-194) (○), Ag2/PRA(19-194) (▪), Ag2/PRA(1-18) DNA (▵), or the pVR1012 vector alone (•), as measured by an ELISA using full-length GST-Ag2/PRA recombinant protein as the target antigen. Results are mean antibody levels in assays of sera pooled from groups of 10 mice 12 days after i.p. challenge with 2,500 arthroconidia. Similar results were obtained in three independent experiments.
Vaccine efficacy of Ag2/PRA(19-194) with TPA signal sequence.
The Ag2/PRA(19-194) DNA construct showed reduced vaccine capacity compared to the full-length Ag2/PRA(1-194) DNA construct. This reduced efficacy could be attributable, in part, to the fact that the truncated Ag2/PRA(19-194) DNA and the pVR1012 vector used to deliver Ag2/PRA(19-194) DNA lacked a signal sequence. Absent a signal sequence, the intracellular processing may be altered or the translated peptide may not be secreted (or secreted at diminished levels) and, as a consequence, have reduced exposure to antigen-presenting cells. An experiment was done, therefore, to examine the effect of using the pVR1020 vector, which contains the TPA signal sequence (11, 24), for delivering the Ag2/PRA(19-194) DNA. As shown in Fig. 8, mice immunized with Ag2/PRA(19-194) ligated to pVR1020 were significantly more protected than mice immunized with Ag2/PRA(19-194) ligated to pVR1012. In fact, the protective capacity of the pVR1020-Ag2/PRA(19-194) DNA construct was comparable to that obtained with the full-length Ag2/PRA(1-194) DNA.
FIG. 8.
Effect of TPA leader sequence on the vaccine capacity of Ag2/PRA(19-194) DNA. Results are means ± standard errors for groups of 10 mice immunized with Ag2/PRA(1-194) and Ag2/PRA(19-194) DNA in pVR1012 or Ag2/PRA(19-194) DNA in pVR1020 and sacrificed 12 days after i.p. challenge with 2,500 arthroconidia.
DISCUSSION
The main finding of this study is that the signal peptide sequence of C. immitis Ag2/PRA can induce protective immunity against this fungal pathogen. The protective immunity induced by the signal peptide gene alone was comparable to that induced by the full-length Ag2/PRA gene vaccine, as evidenced by the decreased fungal loads in spleen and lung after i.p. challenge with a lethal dose of C. immitis and increased survival over a 40-day period postinfection. The protective immunity induced by the signal sequence was associated with the induction of IFN-γ and was not associated with the production of anti-Coccidioides IgG antibody. In contrast to the vaccine efficacy of the Ag2/PRA(1-18) gene vaccine, the truncated Ag2/PRA(19-194) DNA vaccine, which lacks the signal sequence, was much less effective in inducing protection and IFN-γ but did induce anti-Coccidioides IgG, at levels similar to those induced by the full-length Ag2/PRA gene vaccine. Expression of Ag2/PRA(19-194) DNA fused to the TPA signal sequence significantly enhanced its protective capacity, most likely as a result of increased secretion with elevated uptake by antigen-processing cells.
Although signal sequences have been recently linked to the vaccine-induced immune responses (3, 16, 14, 18), investigations have not reported that immunization with signal sequences alone can induce protective immunity. In an earlier study (14), Haddad and coworkers showed that DNA vaccines containing and lacking a signal sequence induced differential induction of IgG subclasses. In those studies, serum IgG1 responses predominated when mice were immunized with signal sequence-containing DNA vaccine, while mixed IgG1-IgG2a profiles were obtained in mice given the DNA vaccine lacking signal sequence. Similarly, in a separate study, Drew et al. (12) found that the secreted form of DNA vaccine Taenia ovis 45W, which contains signal sequence, induced a stronger IgG response in BALB/c mice than did the DNA vaccine lacking signal sequence. In an animal model of tuberculosis, Baldwin et al. (3) found that in addition to the effects on humoral immune response, immunization with Ag85A DNA vaccine containing the signal sequence induced a stronger protective immunity against tuberculosis than immunization with Ag85A DNA lacking signal sequence. Similar findings were reported by other investigators in studies of an Ag85B DNA vaccine (18). While these investigators showed that the signal sequence could play an important role in modifying DNA vaccine-mediated protective immunity, they did not directly examine the protective capacity or immunogenicity of the signal sequence alone. In this investigation, we showed that the signal sequence alone could protect mice against challenge and that the protective capacity of the signal sequence correlated with the induction of IFN-γ.
The Ag2/PRA(1-18) (signal sequence) DNA vaccine induced protection against C. immitis, at a level comparable to that obtained with the full-length gene, but differed from the latter in that the signal sequence vaccine did not induce detectable antibody. This lack of antibody detection is not attributable to the absence of the N-terminal signal sequence region on the Ag2/PRA fusion protein used as a target antigen in the ELISA. That is, the gene encoding the GST peptide was inserted upstream of Ag2/PRA cDNA and hence was expressed on the N terminus preceding the signal peptide sequence (35). The assumption that the signal peptide lacks a B-cell-reactive epitope is supported by an earlier finding that sera from patients with active coccidioidomycosis recognized the recombinant GST-Ag2/PRA(1-194) fusion protein in immunoblots but did not react with a recombinant GST-Ag2/PRA(1-18) peptide (34). The protective capacity of Ag2/PRA(1-18) DNA in the absence of a detectable antibody response is consistent with earlier reports that antibodies do not appear to have a protective role in host resistance to C. immitis (6, 9).
We have previously reported that the production of IFN-γ is a surrogate marker of host resistance to C. immitis (9, 15-17, 25). Native, recombinant, and gene vaccines of C. immitis that were shown to protect against challenge with this fungus have consistently induced IFN-γ, and conversely, vaccine preparations that failed to induce protection did not induce this cytokine. While it has been widely assumed that the IFN-γ response in coccidioidomycosis represents activation of T-helper 1 cells, it may be that it is also associated with the activation of cytotoxic T lymphocytes (CTL). In support of this possibility, a database search of published major histocompatibility complex (MHC) motifs (30) revealed a high probability that the translated Ag2/PRA(1-18) signal sequence contains an MHC class I binding motif. Although CTL activity in coccidioidomycosis has not been reported, a role for CD8+ T cells in host defense is suggested by the finding that optimal protection by adoptive transfer of immune spleen cells to syngeneic mice was obtained when both CD4 and CD8 cells were present; that is, depletion of either subset population reduced the level of protection conferred to recipient mice (9). The results of the present study further predict that the IFN-γ-inducing epitope on Ag2/PRA(1-18) differs from that on Ag2/PRA(19-194). This prediction is made on the basis that the two constructs do not share sequence homology, yet both induce IFN-γ, and the sum of the IFN-γ levels induced by the two constructs approximates that induced by the full-length Ag2/PRA(1-194) gene. This concept is further supported by the finding that splenocytes from mice immunized with the Ag2/PRA(1-18) signal sequence or the full-length Ag2/PRA DNA but not Ag2/PRA(19-194) produced IFN-γ in response to stimulation with the Ag2/PRA(1-18) synthetic peptide. Delineating the nature and location of the IFN-γ-inducing epitopes of Ag2/PRA and the T-cell population(s) which is activated have important implications in vaccine development.
MHC class I binding activity has been recently recognized as the most important function of signal peptides for in vivo processing and antigen presentation during DNA immunization. Our results showed that Ag2/PRA(1-18) DNA vaccine, which is predicted to have MHC class I binding activity, induced protective immunity, whereas Ag2/PRA(M1-18), which contains a frameshift mutation, was nonprotective and lacked predictable MHC class I binding activity. Therefore, it seems reasonable to predict that the CD8+ CTL would be an important component for the signal gene vaccine's efficacy in inducing protective immunity. Further studies are clearly warranted to examine the role of CTL in host defense against C. immitis and the capacity of the Ag2/PRA(1-18) DNA vaccine to induce this response.
In addition to functioning as antigen epitope to induce protective immunity, the Ag2/PRA signal sequence also functions as secretion signal to transport native Ag2/PRA protein out of cells to stimulate immune responses (26). Ag2/PRA(19-194) DNA, which lacked a signal sequence, was less protective than Ag2/PRA(1-194) DNA. The addition of the TPA signal sequence to Ag2/PRA(19-194) DNA significantly potentiated the vaccine capacity of the Ag2/PRA(19-194) DNA, to a level that was comparable to the protection induced by the full-length Ag2/PRA gene vaccine. These results are concordant with reports by Li et al. (24) and Delogu et al. (11), who showed that fusion of Mycobacterium tuberculosis DNA vaccine candidates with the TPA signal sequence increased their expression as secreted proteins and significantly enhanced their immunogenicity in mice.
In summary, our results indicate that Ag2/PRA signal gene sequence alone is an effective vaccine candidate in our animal model of coccidioidomycosis. This is the first study to show that the signal sequence of a C. immitis gene is able to induce protective immunity against challenge with this fungal pathogen. This investigation demonstrates that the role of signal sequence is not only to transport mature protein but more importantly to function as an immunoepitope in the induction of immune response. We believe that these results are encouraging and warrant further studies of the role of the Ag2/PRA signal sequence in the induction of protective immunity against C. immitis. It will be of paramount importance to modify the Ag2/PRA gene vaccine or secreted peptide so as to induce protection against pulmonary challenge. While genetic vaccination with Ag2/PRA is effective against i.p. challenge (1, 15, 19, 17), mice challenged via a pulmonary route show a reduction in the fungal load in their livers and spleens at 12 days postinfection but not in their lungs, and their survival rate is no different than that observed in control mice at days 1 through 30 postinfection (15, 17). The relative inefficacy of the Ag2/PRA vaccine and derived constructs against pulmonary challenge is a major limitation and clearly shows the need for studies to optimize vaccine delivery for protection at the lung level.
Acknowledgments
This work was supported by a grant from the California HealthCare Foundation, the Department of Health Services of the State of California, and California State University, Bakersfield.
We thank Adrian Donias, Jerileigh Lopez, Melanie Woitaske, Heping Jiang, and Qinghong Shi for their expert technical assistance.
Editor: T. R. Kozel
REFERENCES
- 1.Abuodeh, R. O., L. F. Shubitz, E. Siegel, S. Snyder, T. Peng, K. I. Orsborn, E. Brummer, D. A. Stevens, and J. N. Galgiani. 1999. Resistance to Coccidioides immitis in mice after immunization with recombinant protein or a DNA vaccine of a proline-rich antigen. Infect. Immun. 67:2935-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ampel, N. M., G. C. Bejarano, S. D. Salas, and J. N. Galgiani. 1992. In vitro assessment of cellular immunity in human coccidioidomycosis: relationship between dermal hypersensitivity, lymphocyte transformation, and lymphokine production by peripheral blood mononuclear cells from healthy adults. J. Infect. Dis. 165:710-715. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin, S. L., C. D. D'Souza, I. M. Orme, M. A. Liu, L. Huygen, O. Denis, A. Tang, L. Zhu, D. Montgomery, and J. B. Ulmer. 1999. Immunogenicity and protective efficacy of DNA vaccines encoding secreted and non-secreted forms of Mycobacterium tuberculosis Ag85A. Tuber. Lung Dis. 79:251-259. [DOI] [PubMed] [Google Scholar]
- 4.Corry, D. B., N. M. Ampel, L. Christian, R. M. Locksley, and J. N. Galgiani. 1996. Cytokine production by peripheral blood mononuclear cells in human coccidioidomycosis. J. Infect. Dis. 174:440-443. [DOI] [PubMed] [Google Scholar]
- 5.Cox, R. A. 1989. Antigenic structure of Coccidioides immitis, p. 133-170. In E. Kurstak, G. Marquis, P. Auger, L. De Repentigny, and S. Montplaisir (ed.), Immunology of fungal diseases. Marcel Dekker, Inc., New York, N.Y.
- 6.Cox, R. A. 1993. Coccidioidomycosis, p. 173-211. In J. W. Murphy, H. Friedman, and M. Bendinelli (ed.), Fungal infections and immune responses. Plenum Press, New York, N.Y.
- 7.Cox, R. A., E. Brummer, and G. Lecara. 1977. In vitro lymphocyte responses of coccidioidin skin-test positive and -negative persons to coccidioidin, spherulin, and a Coccidioides immitis cell wall antigen. Infect. Immun. 15:751-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox, R. A., W. Kennell, L. Boncyk, and J. W. Murphy. 1988. Induction and expression of cell-mediated immune responses in inbred mice infected with Coccidioides immitis. Infect. Immun. 56:13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox, R. A., and D. M. Magee. 1998. Protective immunity in coccidioidomycosis. Res. Immunol. 149:417-428. [DOI] [PubMed] [Google Scholar]
- 10.Cox, R. A., and J. R. Vivas. 1977. Spectrum of in vivo and in vitro immune responses in coccidioidomycosis. Cell. Immunol. 31:130-141. [DOI] [PubMed] [Google Scholar]
- 11.Delogu, G., A. Li, C. Repique, F. Collins, and S. L. Morris. 2002. DNA vaccine combinations expressing either tissue plasminogen activator signal sequence fusion proteins or ubiquitin-conjugated antigens induce sustained protective immunity in a mouse model of pulmonary tuberculosis. Infect. Immun. 70:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drew, D. R., M. Lightowlers, and R. A. Strugnell. 2000. Humoral immune response to DNA vaccines expressing secreted membrane bound and non-secreted forms of the Taenia ovis 45W antigen. Vaccine 18:2522-2532. [DOI] [PubMed] [Google Scholar]
- 13.Dugger, K. O., K. M. Villareal, A. Ngyuen, C. R. Zimmermann, J. H. Law, and J. N. Galgiani. 1996. Cloning and sequence analysis of the cDNA for a protein from Coccidioides immitis with immunogenic potential. Biochem. Biophys. Res. Commun. 218:485-489. [DOI] [PubMed] [Google Scholar]
- 14.Haddad, D., S. Liljeqvist, S. Stahl, P. Perlmann, K. Berzins, and N. Ahlborg. 1998. Differential induction of immunoglobulin G subclasses by immunization with DNA vectors containing or lacking a signal sequence. Immunol. Lett. 61:201-204. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, C., D. M. Magee, T. N. Quitugua, and R. A. Cox. 1999. Genetic vaccination against Coccidioides immitis: comparison of vaccine efficacy of recombinant antigen 2 and antigen 2 cDNA. Infect. Immun. 67:630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang, C., D. M. Magee, and R. A. Cox. 1999. Construction of a single chain interleukin-12-expressing retroviral vector and its application in cytokine gene therapy against experimental coccidioidomycosis. Infect. Immun. 67:2996-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang, C., D. M. Magee, and R. A. Cox. 1999. Coadministration of interleukin-12 expression vector with antigen 2 cDNA enhances induction of protective immunity against Coccidioides immitis. Infect. Immun. 67:5848-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamath, A. T., C. G. Feng, M. Macdonald, H. Briscoe, and W. J. Britton. 1999. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 67:1702-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkland, T. N., F. Finley, K. I. Orsborn, and J. N. Galgiani. 1998. Evaluation of the proline-rich antigen of Coccidioides immitis as a vaccine candidate in mice. Infect. Immun. 66:3519-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong, Y. M., H. B. Levine, and C. E. Smith. 1963. Immunogenic properties of nondisrupted and disrupted spherules of Coccidioides immitis in mice. Sabouraudia 2:131-142. [PubMed] [Google Scholar]
- 21.Lecara, G., R. A. Cox, and R. B. Simpson. 1983. Coccidioides immitis vaccine: potential of an alkali-soluble, water-soluble cell wall antigen. Infect. Immun. 39:473-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine, H. B., J. M. Cobb, and C. E. Smith. 1961. Immunogenicity of spherule-endospore vaccines of Coccidioides immitis for mice. J. Immunol. 87:218-227. [PubMed] [Google Scholar]
- 23.Levine, H. B., Y. C. M. Kong, and C. E. Smith. 1965. Immunization of mice to Coccidioides immitis: dose, regimen and spherulation stage of killed spherule vaccines. J. Immunol. 94:132-142. [PubMed] [Google Scholar]
- 24.Li, Z., A. Howard, C. Kelley, G. Delogu, F. Collins, and S. Morris. 1999. Immunogenicity of DNA vaccine expressing tuberculosis proteins fused to tissue plasminogen activator signal sequence. Infect. Immun. 67:4780-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magee, D. M., and R. A. Cox. 1995. Roles of gamma interferon and interleukin-4 in genetically determined resistance to Coccidioides immitis. Infect. Immun. 63:3514-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martoglio, B., and B. Dobberstein. 1998. Signal sequence: more than just greasy peptides. Trends Cell Biol. 8:410-415. [DOI] [PubMed] [Google Scholar]
- 27.Pappagianis, D. 1980. Epidemiology of coccidioidomycosis, p. 63. In D. A. Steven (ed.), Coccidioidomycosis. Plenum Publishing Corporation, New York, N.Y.
- 28.Pappagianis, D. 1994. Marked increase in cases of coccidioidomycosis in California: 1991, 1992, and 1993. Clin. Infect. Dis. 19:S14-S18. [DOI] [PubMed] [Google Scholar]
- 29.Pappagianis, D., R. Hector, H. B. Levine, and M. S. Collins. 1979. Immunization of mice against coccidioidomycosis with a subcellular vaccine. Infect. Immun. 25:440-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker, K. C., M. A. Bednarek, and J. E. Coligan. 1994. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J. Immunol. 152:163.. [PubMed] [Google Scholar]
- 31.Patek, P. Q., J. L. Collians, and M. Cohn. 1978. Transformed cell lines susceptible or resistant to in vivo surveillance against tumorigenesis. Nature 276:510-511. [DOI] [PubMed] [Google Scholar]
- 32.Ronaghy, A., B. J. Prakken, K. Takabayashi, G. S. Firestein, D. Boyle, N. J. Zvailfler, S. T. A. Roord, S. Albani, D. A. Carson, and E. Raz. 2002. Immunostimulatory DNA sequences influence the course of adjuvant arthritis. J. Immunol. 168:51-56. [DOI] [PubMed] [Google Scholar]
- 33.Stevens, D. A. 1995. Current concepts: coccidioidomycosis. N. Engl. J. Med. 332:1077-1082. [DOI] [PubMed] [Google Scholar]
- 34.Zhu, Y., V. Tryon, D. M. Magee, and R. A. Cox. 1997. Identification of a Coccidioides immitis antigen 2 domain that expresses B-cell-reactive epitopes. Infect. Immun. 65:3376-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu, Y., C. Yang, D. M. Magee, and R. A. Cox. 1996. Molecular cloning and characterization of Coccidioides immitis antigen 2 cDNA. Infect. Immun. 64:2695-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu, Y., C. Yang, D. M. Magee, and R. A. Cox. 1996. Coccidioides immitis antigen 2: analysis of gene and protein. Gene 181:121-125. [DOI] [PubMed] [Google Scholar]