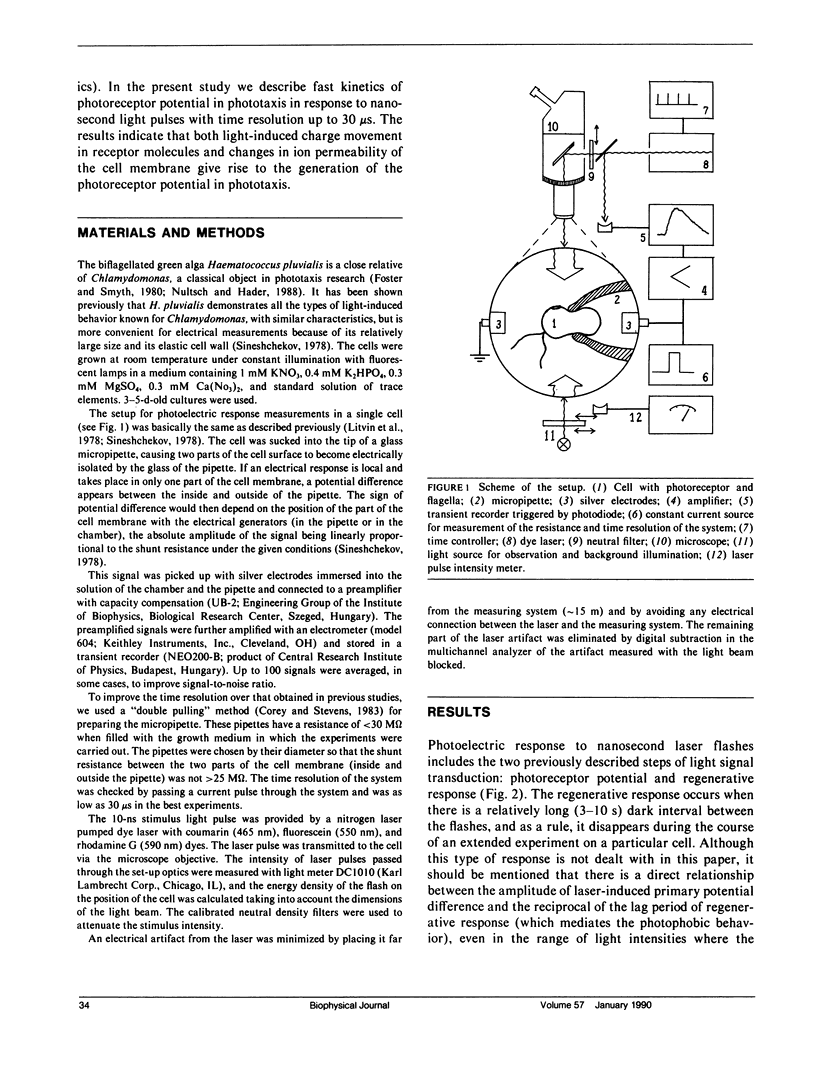
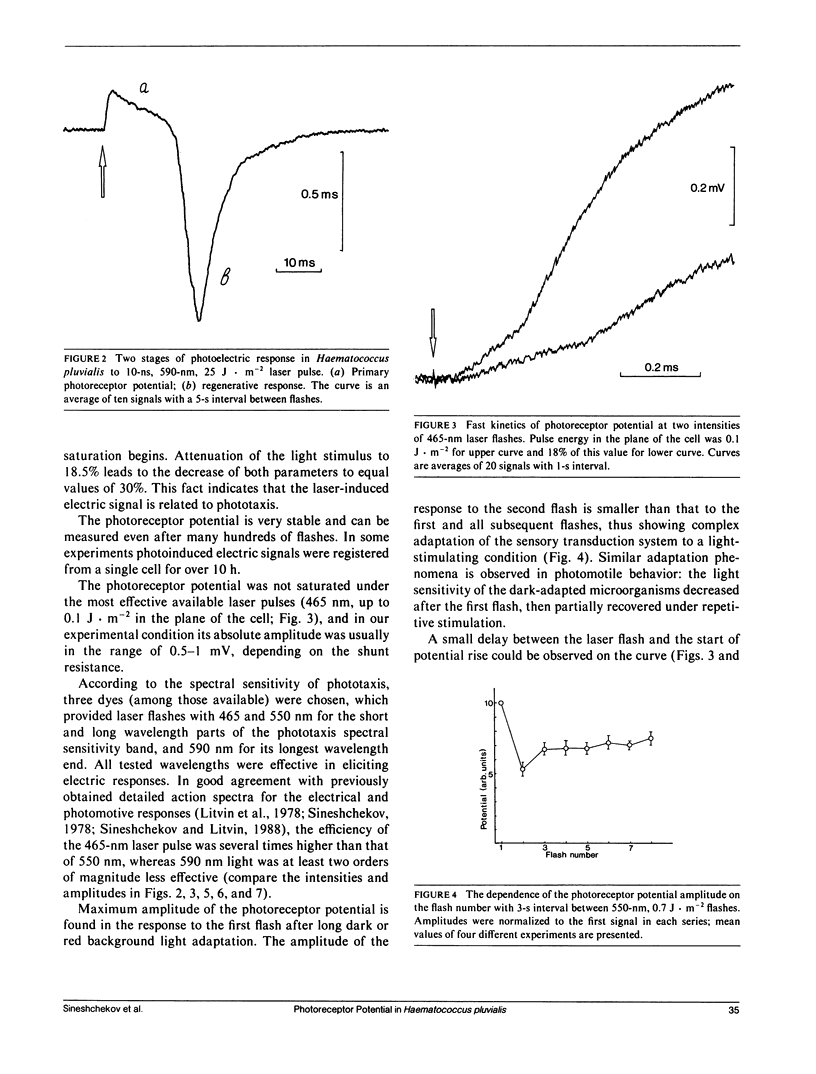
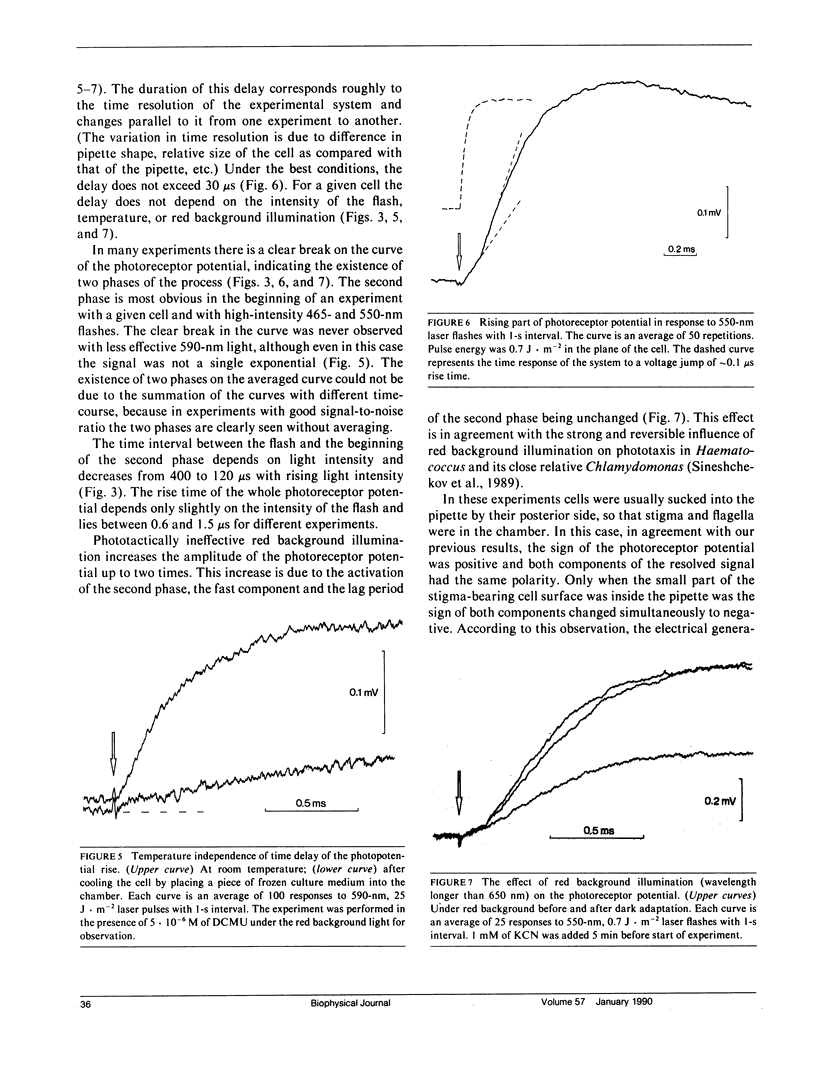
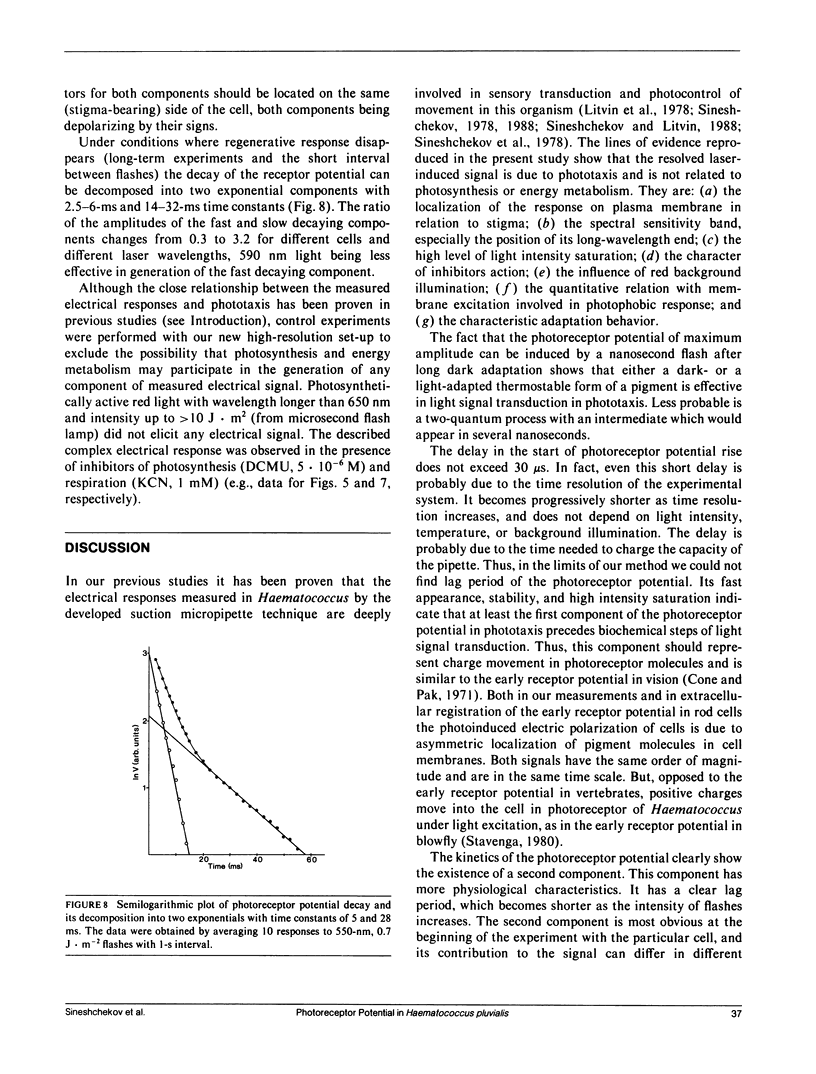
Abstract
The kinetics of the photoreceptor potential of phototaxis in biflagellated green alga Haematococcus pluvialis in response to a 10-ns laser pulse of three wavelengths (465, 550, and 590 nm) were measured in single cells with 30 μs time resolution. The rise and the decay of photoinduced potential are both at least biphasic. The first component of the rise is very stable and has no measurable (<30 μs) time delay. The second component is triggered after a 120-400-μs lag period, depending on flash intensity. Its appearance is sensitive to the physiological state of the cell and the amplitude can be increased by phototactically ineffective red background illumination. The electrical generators for both components are localized in the same region of the cell membrane (on the stigma-bearing side) and these components have the same depolarizing sign. The results indicate that the photoreceptor potential in phototaxis comprises two components, which could be interpreted as light-induced charge movement within the photoreceptor molecules and changes in ion permeability of the cell membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Foster K. W., Saranak J., Patel N., Zarilli G., Okabe M., Kline T., Nakanishi K. A rhodopsin is the functional photoreceptor for phototaxis in the unicellular eukaryote Chlamydomonas. Nature. 1984 Oct 25;311(5988):756–759. doi: 10.1038/311756a0. [DOI] [PubMed] [Google Scholar]
- Foster K. W., Smyth R. D. Light Antennas in phototactic algae. Microbiol Rev. 1980 Dec;44(4):572–630. doi: 10.1128/mr.44.4.572-630.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegemann P., Hegemann U., Foster K. W. Reversible bleaching of Chlamydomonas reinhardtii rhodopsin in vivo. Photochem Photobiol. 1988 Jul;48(1):123–128. doi: 10.1111/j.1751-1097.1988.tb02796.x. [DOI] [PubMed] [Google Scholar]
- Litvin F. F., Sineshchekov O. A., Sineshchekov V. A. Photoreceptor electric potential in the phototaxis of the alga Haematococcus pluvialis. Nature. 1978 Feb 2;271(5644):476–478. doi: 10.1038/271476a0. [DOI] [PubMed] [Google Scholar]
- Nultsch W., Häder D. P. Photomovement in motile microorganisms--II. Photochem Photobiol. 1988 Jun;47(6):837–869. doi: 10.1111/j.1751-1097.1988.tb01668.x. [DOI] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Sensory rhodopsins of halobacteria. Annu Rev Biophys Biophys Chem. 1988;17:193–215. doi: 10.1146/annurev.bb.17.060188.001205. [DOI] [PubMed] [Google Scholar]



