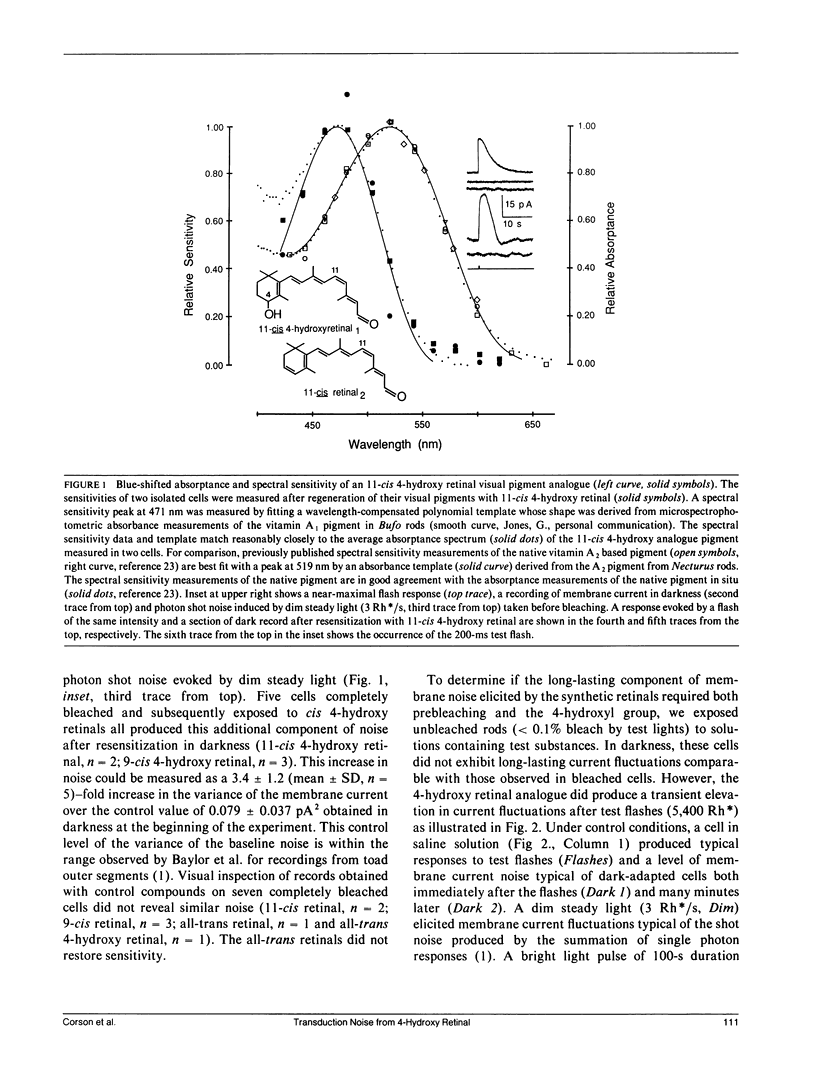
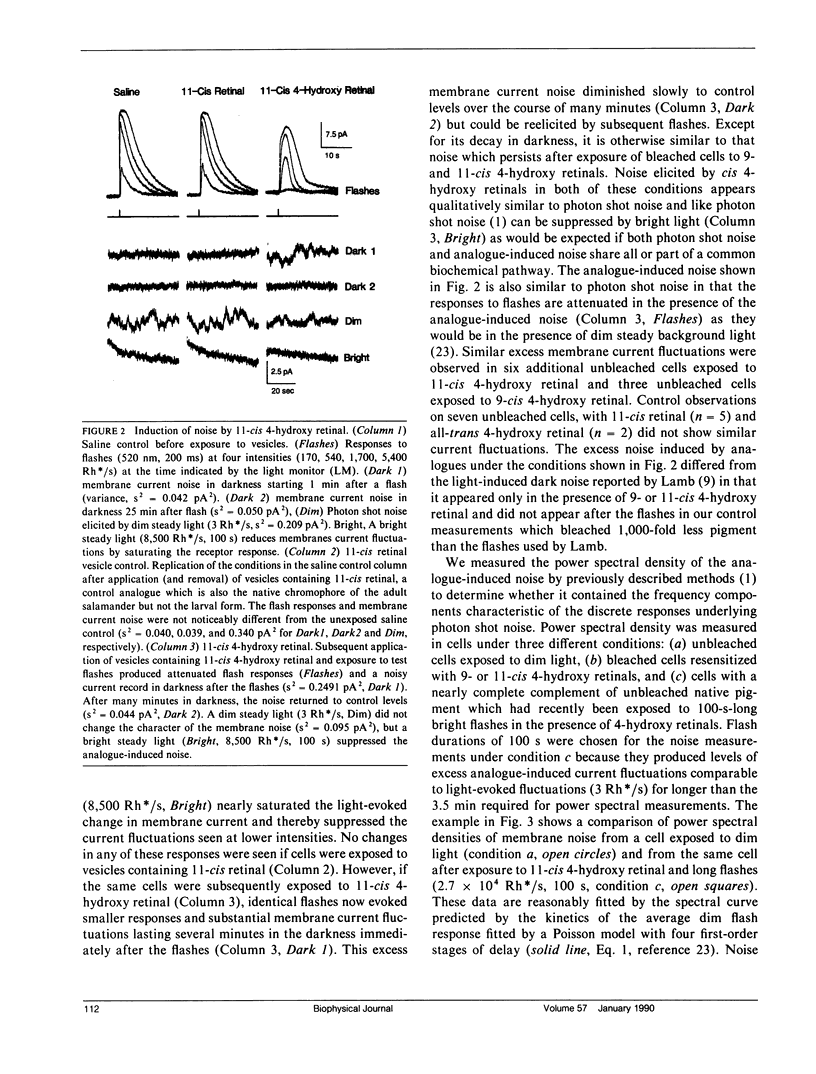
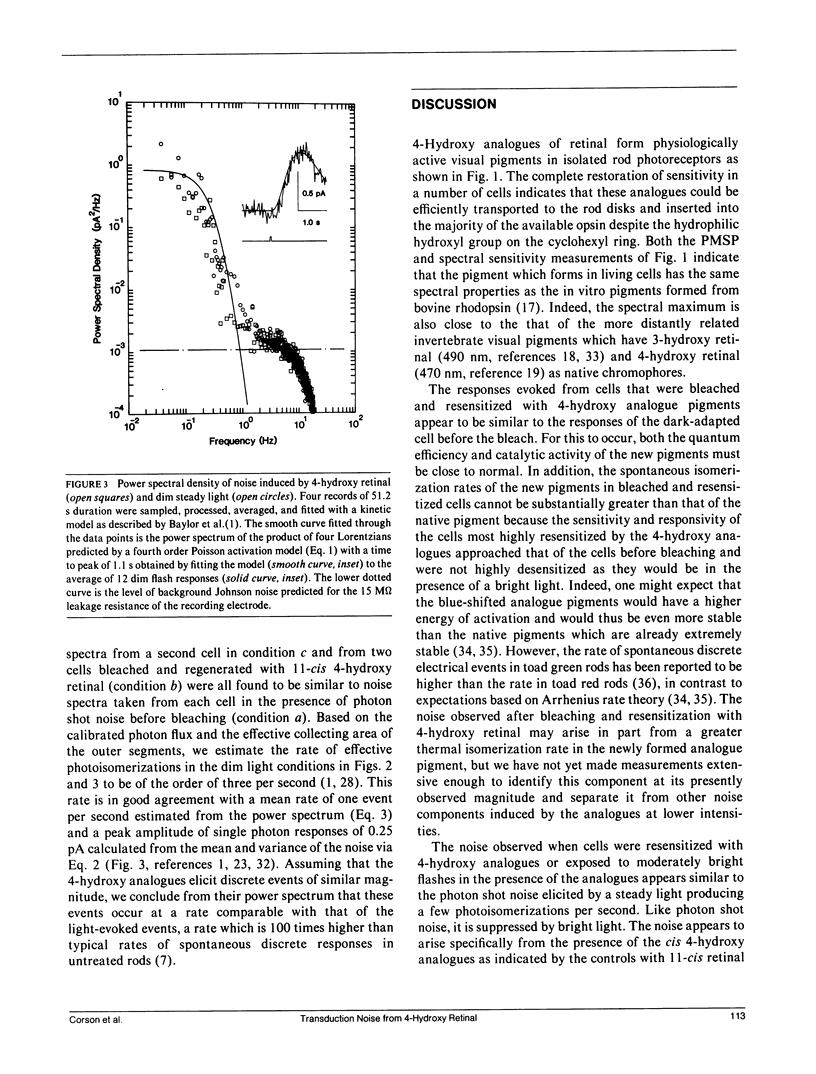
Abstract
New visual pigments were formed with 4-hydroxy retinals in isolated vertebrate rod photoreceptors by exposing bleached rods from the tiger salamander, Ambystoma tigrinum, to lipid vesicles containing the analogues. Formation of physiologically active pigment was demonstrated by the restoration of sensitivity and by a shift of approximately 50 nm in the peak of both the visual pigment absorptance spectrum and rod spectral sensitivity spectrum from approximately 520 to approximately 470 nm for 11-cis 4-hydroxy retinal. Membrane current recordings from the inner segments of isolated rods revealed excess fluctuations in membrane current after formation of the new pigment in bleached cells or after exposure of unbleached cells to flashes in the presence of the analogue. The excess current fluctuations are similar to the fluctuations elicited by steady light producing a few discrete responses per second, a rate approximately 100 times greater than the normal rate of spontaneous events in darkness. These results suggest that analogues of retinal can produce alterations in the frequency of production of discrete responses in darkness in rod photoreceptors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F., Falk G. Dark noise in retinal bipolar cells and stability of rhodopsin in rods. Nature. 1977 Nov 3;270(5632):69–71. doi: 10.1038/270069a0. [DOI] [PubMed] [Google Scholar]
- Ashmore J. F., Falk G. Photon-like signals following weak rhodopsin bleaches. Nature. 1981 Feb 5;289(5797):489–491. doi: 10.1038/289489a0. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B. Purkinje shift and retinal noise. Nature. 1957 Feb 2;179(4553):255–256. doi: 10.1038/179255b0. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A. Photoreceptor signals and vision. Proctor lecture. Invest Ophthalmol Vis Sci. 1987 Jan;28(1):34–49. [PubMed] [Google Scholar]
- Chabre M. Trigger and amplification mechanisms in visual phototransduction. Annu Rev Biophys Biophys Chem. 1985;14:331–360. doi: 10.1146/annurev.bb.14.060185.001555. [DOI] [PubMed] [Google Scholar]
- Cornwall M. C., MacNichol E. F., Jr, Fein A. Absorptance and spectral sensitivity measurements of rod photoreceptors of the tiger salamander, Ambystoma tigrinum. Vision Res. 1984;24(11):1651–1659. doi: 10.1016/0042-6989(84)90323-7. [DOI] [PubMed] [Google Scholar]
- Crouch R. K. Studies of rhodopsin and bacteriorhodopsin using modified retinals. Photochem Photobiol. 1986 Dec;44(6):803–807. doi: 10.1111/j.1751-1097.1986.tb05540.x. [DOI] [PubMed] [Google Scholar]
- Gilbard J. P., Gray K. L., Rossi S. R. Improved technique for storage of tear microvolumes. Invest Ophthalmol Vis Sci. 1987 Feb;28(2):401–403. [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D. Sources of noise in photoreceptor transduction. J Opt Soc Am A. 1987 Dec;4(12):2295–2300. doi: 10.1364/josaa.4.002295. [DOI] [PubMed] [Google Scholar]
- Lamb T. D. Spontaneous quantal events induced in toad rods by pigment bleaching. Nature. 1980 Sep 25;287(5780):349–351. doi: 10.1038/287349a0. [DOI] [PubMed] [Google Scholar]
- Lamb T. D. The involvement of rod photoreceptors in dark adaptation. Vision Res. 1981;21(12):1773–1782. doi: 10.1016/0042-6989(81)90211-x. [DOI] [PubMed] [Google Scholar]
- Lisman J. The role of metarhodopsin in the generation of spontaneous quantum bumps in ultraviolet receptors of Limulus median eye. Evidence for reverse reactions into an active state. J Gen Physiol. 1985 Feb;85(2):171–187. doi: 10.1085/jgp.85.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S., Seidou M., Uchiyama I., Sekiya N., Hiraki K., Yoshihara K., Kito Y. 4-Hydroxyretinal, a new visual pigment chromophore found in the bioluminescent squid, Watasenia scintillans. Biochim Biophys Acta. 1988 Sep 8;966(3):370–374. doi: 10.1016/0304-4165(88)90087-6. [DOI] [PubMed] [Google Scholar]
- Matthews G. Dark noise in the outer segment membrane current of green rod photoreceptors from toad retina. J Physiol. 1984 Apr;349:607–618. doi: 10.1113/jphysiol.1984.sp015176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G. Physiological characteristics of single green rod photoreceptors from toad retina. J Physiol. 1983 Sep;342:347–359. doi: 10.1113/jphysiol.1983.sp014855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroy S. E. Characteristics of Drosophila rhodopsin in wild-type and norpA vision transduction mutants. J Gen Physiol. 1978 Nov;72(5):717–732. doi: 10.1085/jgp.72.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman J. I., Nodes B. R., Pepperberg D. R. Utilization of retinoids in the bullfrog retina. J Gen Physiol. 1982 Dec;80(6):885–913. doi: 10.1085/jgp.80.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renk G., Crouch R. K. Analogue pigment studies of chromophore-protein interactions in metarhodopsins. Biochemistry. 1989 Jan 24;28(2):907–912. doi: 10.1021/bi00428a075. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Wald G. Molecular basis of visual excitation. Science. 1968 Oct 11;162(3850):230–239. doi: 10.1126/science.162.3850.230. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Matthews G., Baylor D. A. Thermal activation of the visual transduction mechanism in retinal rods. Nature. 1979 Jun 28;279(5716):806–807. doi: 10.1038/279806a0. [DOI] [PubMed] [Google Scholar]
- Yoshikami S., Nöll G. N. Technique for introducing retinol analogs into the isolated retina. Methods Enzymol. 1982;81:447–451. doi: 10.1016/s0076-6879(82)81062-8. [DOI] [PubMed] [Google Scholar]


