Abstract
Vaccination with heat shock protein 60 (Hsp60) from Histoplasma capsulatum induces a protective immune response in mice. We explored the cellular and molecular requirements for the efficacy of recombinant Hsp60 in mice. Depletion of CD4+, but not CD8+, cells during the inductive phase of vaccination abolished protection, as assessed by survival and by the fungal burden in lungs and spleens. In the expressive phase, the elimination of CD4+ or CD8+ cells after immunization did not significantly alter fungal recovery or survival from a lethal challenge. Depletion of both subpopulations after Hsp60 vaccination resulted in a failure to control a lethal infection and a higher fungal burden in lungs and spleens. Cytokine release by spleen cells from mice vaccinated with Hsp60 produced substantially more gamma interferon and interleukin-10 and -12 than that of cells from mice immunized with either H. capsulatum recombinant Hsp70 or bovine serum albumin. The generation of gamma interferon, but not of interleukin-10, was dependent on T cells, in particular CD4+ cells. Treatment of Hsp60-immunized mice with monoclonal antibody to gamma interferon or interleukin-10 or -12 in the inductive phase of vaccination was accompanied by increased recovery of yeast cells from lungs and spleens and 100% mortality. Likewise, the neutralization of gamma interferon or interleukin-12 abolished the protective effect of Hsp60 in the expressive phase. These results delineate the complexity of the regulatory elements necessary for vaccination against this fungus.
Infection with the dimorphic, pathogenic fungus Histoplasma capsulatum produces an array of clinical symptoms ranging from an acute influenza-like illness to a chronic cavitary disease in lungs to a progressive disseminated form that can involve multiple organ systems. Histoplasmosis is one of the most common respiratory mycoses, infecting an estimated 200,000 to 500,000 individuals annually in regions of the midwestern and southeastern United States where it is endemic. Following infection via inhalation of mycelial fragments and microconidia, these fungal elements transform into yeast cells. Although the conversion from the mycelial to the yeast form is a major virulence determinant, yeast cells are the fungal elements that cause the clinicopathological manifestations of histoplasmosis (12).
Restriction of yeast cell growth requires the concerted interaction of T cells and professional phagocytes (6, 36, 39). A principal force in the generation of a protective immune response is the activation of T cells; the resolution of infection is coincident with measurements of T-cell activation such as cytokine release and proliferation (11, 41). The failure of T cells to express effector function is often associated with progressive disease (7, 18). Several protein-containing antigens from the fungus can promote T-cell activation, yet only two are protective in mice when used as prophylactic vaccines. One is heat shock protein 60 (Hsp60), and the other is H antigen (13, 14, 19). Both native and recombinant (r) Hsp60 from H. capsulatum mediate a protective immune response. rHsp60 reduces the fungal burden in mice given a sublethal inoculum of yeast cells and improves survival in animals challenged intranasally (i.n.) with a lethal inoculum (14, 19). The cellular and molecular determinants of the efficacy of rHsp60 have not been defined. Herein, we delineate the regulation of the protective immune response mediated by rHsp60 in the inductive and expressive phases of vaccine-induced immunity. Our findings demonstrate that CD4+ cells, gamma interferon (IFN-γ), interleukin-10 (IL-10), and IL-12 were necessary during the inductive phase whereas both CD4+ and CD8+ cells, IL-12, and IFN-γ were important in the expressive phase.
MATERIALS AND METHODS
Mice.
C57BL/6 mice and athymic nude mice were purchased from the National Cancer Institute (Fredericksburg, Md.). Animals were housed in isolator cages and were maintained by the University of Cincinnati Department of Laboratory Animal Medicine, which is accredited by the American Association for Accreditation of Laboratory Animal Medicine. All animal experiments were done in accordance with the Animal Welfare Act guidelines of the National Institutes of Health.
Preparation of H. capsulatum and infection of mice.
H. capsulatum yeast cells were prepared as described previously (13). To produce infection, animals were infected i.n. with 1.25 × 107 or 2 × 106 H. capsulatum yeast cells in a volume of 30 to 50 μl.
Organ culture for H. capsulatum.
Recovery of H. capsulatum was performed as described previously (13). The fungal burden was expressed as the mean number of CFU per whole organ ± the standard error of the mean (SEM). The limit of detection was 102 CFU.
MAb.
All monoclonal antibodies (MAb) were produced as ascites from athymic nude mice and purified by protein G chromatography (Pierce Chemical Co., Rockford, Ill.). The list of MAb for these studies includes anti-CD4 (clone GK 1.5, rat immunoglobulin G2b [IgG2b]), anti-CD8 (clone 2.43, rat IgG2b), anti-IFN-γ (clone XMG 1.2, rat IgG1), anti-IL-10 (clone JES 2A5, rat IgG2b), and anti-IL-12 (clones C15.1 and C15.6, rat IgG1). The cell lines that produced MAb to IFN-γ and IL-10 were a gift from J. Abrams (DNAX Research Institute, Palo Alto, Calif.), and the cell lines generating MAb to IL-12 were a kind gift of Giorgio Trinchieri (Schering-Plough Co., Dardilly, France). All MAb were assessed for the presence of endotoxin and were found to have <5 ng/mg of protein.
Antigens.
rHsp60 and rHsp70 from H. capsulatum were generated as described previously (4, 19). In brief, the genes were cloned into the NdeI and BamHI sites of pET19b. To express recombinant protein, Escherichia coli harboring the plasmid was grown at 37°C in Luria-Bertani broth until the optical density at 600 nm reached 0.4 to 0.5. Subsequently, isopropyl-thiogalactose was added to the cultures at a final concentration of 1 mM. Cultures were continued for 3 h. Cells were harvested by centrifugation at 5,000 × g. The pellet was suspended in a buffer consisting of 5 mM imidazole, 500 mM NaCl, and 20 mM Tris-Cl (pH 7.9) and lysed by freeze-thaw cycles followed by sonication. Soluble and insoluble fractions were separated by centrifugation at 20,000 × g.
The insoluble pellet was suspended in a buffer consisting of 6 M urea, 500 mM NaCl, 5 mM imidazole, and 20 mM Tris-Cl (pH 7.9). The denatured material was recovered in supernatants after centrifugation at 20,000 × g and then filtered to remove particulate material. The protein was purified by metal chelate chromatography with a Ni2+-Sepharose affinity column (His-Bind; Novagen, Madison, Wis.). The recombinant protein was eluted with the same buffer as described above, except that it contained 1 M imidazole. The eluate was dialyzed against buffer containing decreasing amounts of urea. The eluate was concentrated by ultrafiltration, and the protein concentration was determined. Both proteins contained less than 10 pg of lipopolysaccharide (LPS)/μg of protein. Bovine serum albumin (BSA) was purchased from Sigma-Aldrich (St. Louis, Mo.).
Immunization.
Mice were immunized subcutaneously at the base of the tail with rHsp60, rHsp70, or BSA. Antigens were suspended in adjuvant containing monophosphoryl lipid A, synthetic trehalose dicorynomycolate, and cell wall skeleton (Corixa, Hamilton, Mont.) at a concentration of 1 mg/ml. The cell wall skeleton was derived from streptococcal species. Animals were injected subcutaneously with 0.1 ml of emulsion (100 μg of protein) twice. Injections were separated by 2 weeks.
MAb treatment of mice vaccinated with rHsp60.
To determine the influence of CD4+ or CD8+ cells on the inductive phase of vaccination, mice were injected intraperitoneally with 100 μg of MAb to CD4 or CD8 on days −7 and −3 and on the day of the first immunization. This amount of MAb achieved a ⩾95% depletion of both cell populations as determined by flow cytometry. Subsequently, mice received 100 μg of MAb each week until the last injection. Mice were given 1 mg of MAb to IFN-γ or IL-10 at the time of the first immunization and each week thereafter until the last immunization. To neutralize IL-12 during the inductive phase, mice were administered 500 μg of MAb (250 μg of C15.1 plus 250 μg of C15.6) on days −1, +1, +3, +5, and +7 of vaccination and each week thereafter up to the last vaccination. Mice were challenged with H. capsulatum 3 weeks postvaccination.
To assess the cellular and molecular requirements for the expressive phase of vaccination, mice were immunized with rHsp60, and 3 weeks after the last injection, mice were infected and concomitantly received either 100 μg of MAb to CD4 or CD8 or both or 1 mg of MAb to IFN-γ or IL-10 each week. For IL-12, mice were administered 500 μg of MAb at the time of infection and on day −1 and days +1, +3, +5, and +7 postinfection. Treatment with all MAb was continued each week.
Splenocyte preparation.
Spleens from mice were removed and teased apart between two ground glass slides. Cells were washed three times in Hanks balanced salt solution (BioWhittaker, Walkersville, Md.) and resuspended at a concentration of 2.5 × 106 cells per ml in RPMI medium containing 10% fetal bovine serum, 5 × 10−5 M 2-mercaptoethanol, 1% sodium pyruvate, 1% nonessential amino acids, 2 mM l-glutamine, and 10 μg of gentamicin/ml.
In vitro generation of cytokine-containing supernatants.
Splenocyte suspensions were prepared from C57BL/6 mice immunized with rHsp60, rHsp70, or BSA at 3, 7, and 14 days after each vaccination. One milliliter of suspension was added to each well of a 24-well plate. Cells were exposed to 25 μg of antigen in a volume of 25 μl or were incubated with an equal volume of buffer. The cell suspensions were cultured for 24 h at 37°C in 5% CO2, and the supernatant from cultures was harvested, filter sterilized, and stored at −70°C until assayed. Exploratory studies established that the results with supernatant from 24-h cultures were similar to the results with supernatant from 48-h cultures.
Cytokine measurement.
Commercially available enzyme-linked immunosorbent assay (ELISA) kits were used to measure IFN-γ, IL-4, IL-12p70, tumor necrosis factor alpha (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF) (Endogen, Cambridge, Mass.), and IL-10 (PharMingen, San Diego, Calif.). The data for cytokine levels were expressed as the change in the level of cytokine, determined by subtracting the amount of cytokine detected in medium alone from that found in the supernatant of antigen-stimulated cells.
Statistical analyses.
The log rank test was used to analyze differences in survival; analysis of variance was employed to analyze differences in cytokine production. If the data were not normally distributed, the Mann-Whitney test was used. Dunnet's correction was used for multiple comparisons.
RESULTS
Requirement for CD4+ or CD8+ cells in vaccine-mediated immunity.
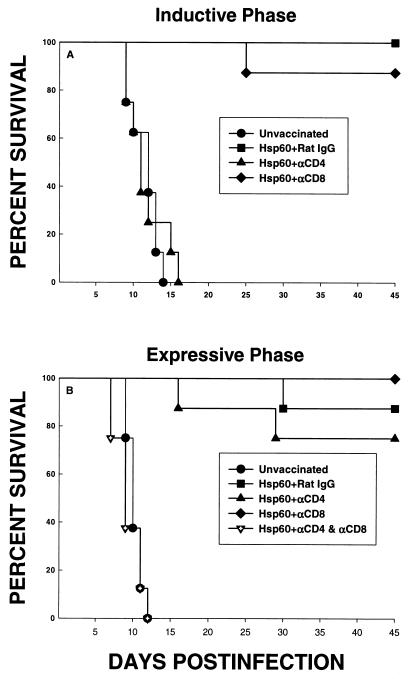
Groups of mice were depleted of either CD4+ or CD8+ cells and immunized with rHsp60. As a control, a group of vaccinated mice was given rat IgG. With the second injection of antigen, treatment with MAb was discontinued. Six weeks later, mice were challenged with 1.25 × 107 yeast cells i.n. The span of time between the termination of vaccination and yeast challenge was selected to permit repopulation of both subpopulations of T cells. Unvaccinated mice failed to survive this challenge. A large proportion of mice vaccinated with rHsp60 and given either rat IgG or MAb to CD8 survived (P < 0.01 compared to unvaccinated mice). In contrast, the elimination of CD4+ cells abolished the protection conferred by rHsp60 (P < 0.01 compared to mice immunized with rHsp60 and given either rat IgG or MAb to CD8) (Fig. 1A).
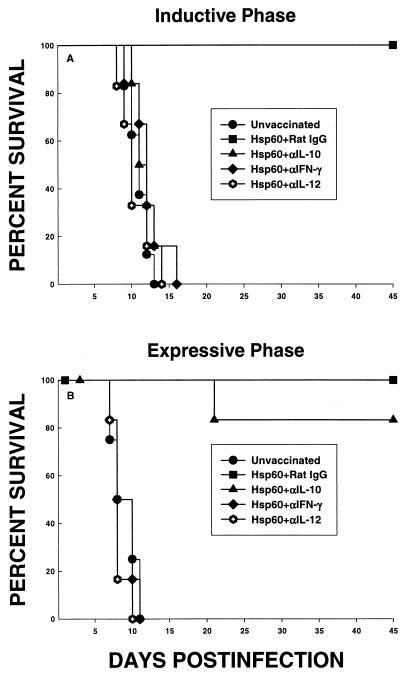
FIG. 1.
Survival of rHsp60-vaccinated mice whose T-cell subsets were eliminated during the inductive (A) or expressive (B) phase. For the inductive phase, groups of mice (n = 8) were immunized with rHsp60 and concomitantly depleted of CD4+ or CD8+ cells. As a control, one vaccinated group received rat IgG. Six weeks later, mice were challenged with 1.25 × 107 yeast cells i.n. For the expressive phase, groups of mice (n = 8) were vaccinated and then depleted of CD4+, CD8+, or CD4+ plus CD8+ cells and challenged with 1.25 × 107 yeast cells. Immunized controls received rat IgG. Survival was monitored for 45 days.
Subsequently, we examined the necessity of CD4+ and/or CD8+ cells for the expressive phase of infection. Mice were vaccinated with rHsp60, and 2 weeks after vaccination, mice were administered MAb to either CD4, CD8, or both or rat IgG and then challenged 1 week after the initiation of cell depletion. All unvaccinated mice succumbed to infection, whereas all vaccinated mice administered rat IgG survived. Depletion of either CD4+ or CD8+ cells did not alter the efficacy of vaccination (Fig. 1B). Vaccinated mice in which both subsets were eliminated failed to control infection (P < 0.01 compared to Hsp60-immunized mice given rat IgG, MAb to CD4, or MAb to CD8). Mortality was 100% in Hsp60-immunized mice deficient in both CD4+ and CD8+ cells (Fig. 1B).
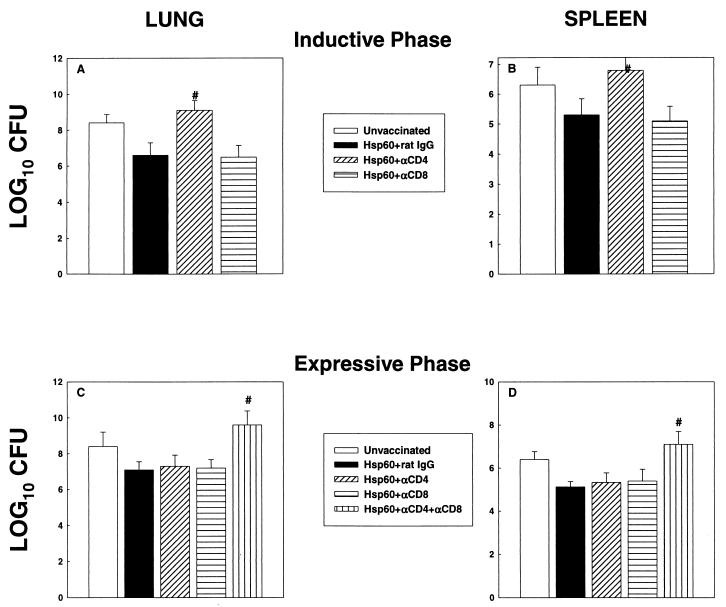
In a separate set of experiments, we determined whether the abolition of rHsp60 efficacy was associated with an increase in the fungal burden in lungs and spleens of mice at day 7 postinfection for both the inductive and expressive phases of vaccination. In the inductive phase, the level of CFU in the lungs and spleens of mice that lacked CD4+ cells was significantly higher (P < 0.01) than that in rHsp60-immunized mice given either rat IgG or MAb to CD8 (Fig. 2A and B). In the expressive phase, the burden was higher (P < 0.01) in the lungs and spleens of mice depleted of both CD4+ and CD8+ cells than in those of vaccinated mice given rat IgG, MAb to CD4, or MAb to CD8 (Fig. 2C and D).
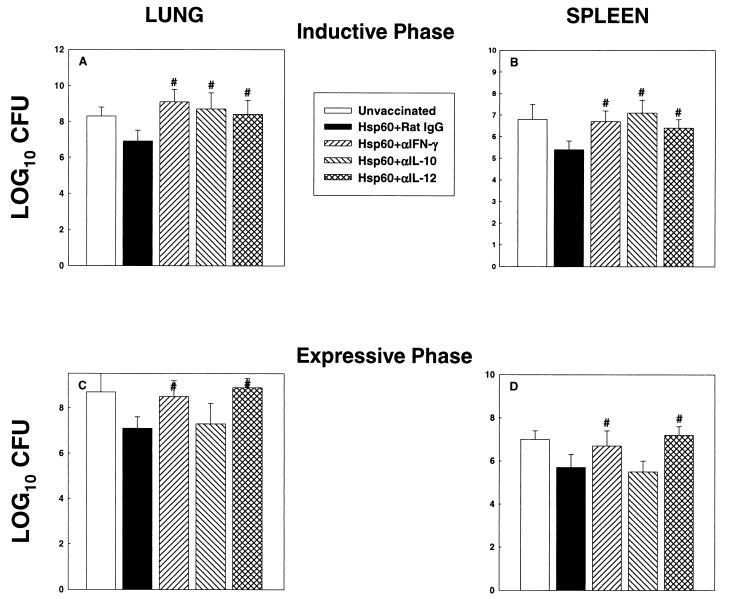
FIG. 2.
CFU in lungs and spleens of T-cell-depleted and rHsp60-vaccinated mice. (A and B) Mice (n = 6) were immunized, concomitantly depleted of CD4+ or CD8+ cells and challenged with 1.25 × 107 yeast cells. A group of immunized mice was administered rat IgG. (C and D) In the expressive phase, groups of mice (n = 6) were vaccinated and then depleted of CD4+, CD8+, or CD4+ plus CD8+ cells challenged with 1.25 × 107 yeast cells. Immunized controls received rat IgG. CFU were enumerated at day 7 of infection. The data represent mean log10 CFU ± SEM. The results of one of two experiments are shown. #, P < 0.01 compared to immunized controls.
Cytokine profiles determined with splenocytes from vaccinated mice.
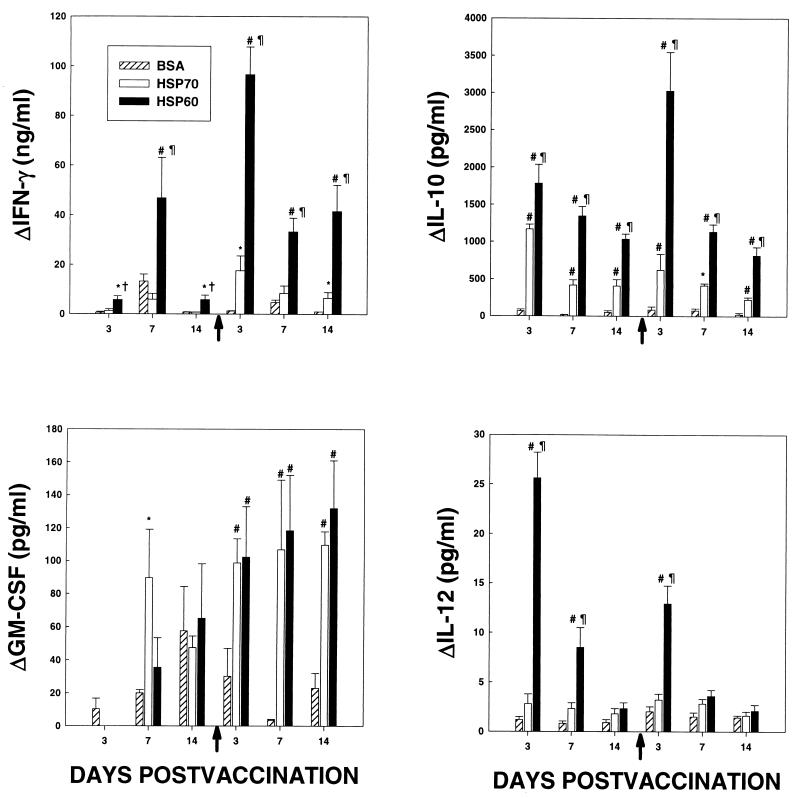
Mice were immunized with rHsp60, rHsp70, or BSA at serial intervals following vaccination; splenocytes were stimulated with antigen, and cytokine levels in supernatants of antigen-stimulated cells were ascertained. rHsp70 was included in these assays since it is known to trigger a cell-mediated immune (CMI) response yet does not mediate a protective response in mice infected with H. capsulatum (12). The production of IFN-γ and IL-10 by splenocytes from mice immunized with rHsp60 was significantly greater (P < 0.01) than that by cells from mice immunized with either rHsp70 or BSA at each time point (Fig. 3). The levels of IFN-γ produced by cells exposed to rHsp70 exceeded (P < 0.05) those produced by BSA-sensitized cells on days 3 and 14 following the second injection of antigen. IL-10 generation by rHsp70-stimulated cells was greater (P < 0.01 to 0.05) than that by BSA-stimulated cells at each day postvaccination. No differences in GM-CSF release were observed among the three groups (Fig. 3). IL-4 and TNF-α levels were assayed concomitantly, and the amount from cells exposed to antigen did not exceed that from cells incubated in medium (data not shown).
FIG. 3.
Cytokine levels in supernatants from splenocytes of mice injected with BSA, rHsp70, or rHsp60 and stimulated with cognate antigen. At serial intervals after immunization, spleen cells from mice were stimulated for 24 h with equal amounts of BSA, rHsp70, or rHsp60, and the levels of cytokines in supernatants were determined by ELISA. Results represent the means ± SEM for six mice at each time point. Arrows along the x axes indicate the time of the second injection of antigen. *, P < 0.05 compared to BSA; #, P < 0.01 compared to BSA; †, P < 0.05 compared to rHsp70; ¶, P < 0.01 compared to rHsp70.
The levels of IL-12 in supernatants of Hsp60-stimulated cells were greater (P < 0.01) than those in supernatants of rHsp70- and BSA-stimulated cells on day 3 after the first and second injections (Fig. 3).
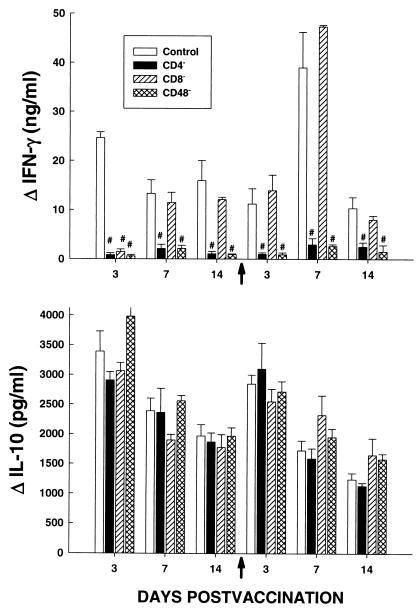
To determine whether the release of IFN-γ and IL-10 by cells from rHsp60-immunized mice was dependent on the presence of T cells, mice were depleted of CD4+, CD8+, or CD4+ plus CD8+ cells and vaccinated with rHsp60. The production of IFN-γ was dramatically reduced by the elimination of CD4+ or CD4+ plus CD8+ cells but not by the depletion of CD8+ cells alone, except on day 3 postvaccination (Fig. 4). The generation of IL-10 was not altered by the elimination of T cells.
FIG. 4.
Contribution of T cells to production of IFN-γ and IL-10. Mice were immunized with rHsp60 and depleted of CD4+ or CD8+ cells or both subsets, and at serial intervals following injection, splenocytes were exposed to rHsp60 or to medium. The levels of cytokines in supernatants from cells were determined by ELISA. The results represent means ± SEM for six mice at each time point. The arrows on the x axes indicate the time of the second injection of antigen. #, P < 0.01 compared to controls.
It is possible that the small amounts of LPS in rHsp60 could have stimulated the release of IL-10 by non-T cells (17). If LPS is responsible for the release of IL-10, its effect should also be observed in naive cells, since this biological action is nonspecific. Splenocytes from naive C57BL/6 mice were incubated with rHsp60 and tested for the IL-10 release. In three experiments, the change in the amount of IL-10 released by splenocytes exposed to rHsp60 for 24 h (18.6 ± 2.9 pg/ml) was not significantly different from that of cells exposed to medium alone (16.9 ± 0.4 pg/ml).
Cytokine regulation of the protective response mediated by rhsp60.
We determined the influence of upregulated IFN-γ, IL-10, and IL-12 on the inductive phase of vaccination. Mice were simultaneously immunized with rHsp60 and given MAb to IFN-γ, IL-10, or IL-12. The administration of MAb was discontinued with the second injection of rHsp60. Three weeks later, mice were challenged with a lethal inoculum of yeast cells. Neutralization of each cytokine abolished the protective immune response conferred by vaccination (Fig. 5A). All unvaccinated mice and those vaccinated with Hsp60 and treated with MAb to IFN-γ, IL-10, or IL-12 died, whereas 100% of Hsp60-immunized mice given rat IgG survived (P < 0.01).
FIG. 5.
Influence of IFN-γ, IL-10, and IL-12 on the efficacy of rHsp60 vaccination. (A) To analyze the role of cytokines in the inductive phase, mice were immunized with rHsp60 and concomitantly treated with MAb to IFN-γ, IL-10, or IL-12 and monitored for survival. (B) For the expressive phase, mice were immunized with rHsp60 and given MAb to IFN-γ, IL-10, or IL-12 3 weeks later. All immunized controls received rat IgG. Mice were challenged with 1.25 × 107 yeast cells and monitored for survival for 45 days.
Inhibition of the activity of IFN-γ and IL-12, but not IL-10, impairs the generation of a protective immune response to primary and secondary H. capsulatum infection (5, 39, 40). Hence, it is plausible that the biologic effect of MAb to IFN-γ or MAb to IL-12 may still be operative in mice 3 weeks after its administration. To exclude this possibility, we injected groups of naive mice with MAb to IFN-γ, MAb to IL-12, or rat IgG each week for 3 consecutive weeks and waited 3 weeks until infecting them. Mice were challenged with 2 × 106 yeast cells i.n. In the absence of biologically active IFN-γ or IL-12, this otherwise sublethal infection becomes a lethal infection (5, 40). As a control, a group of mice received MAb to IFN-γ or MAb to IL-12 at the time of infection and each week thereafter. The lungs and spleens of mice administered MAb to IFN-γ for 3 consecutive weeks and then rested contained a similar number of CFU as those of mice given rat IgG (Table 1). The mice treated with MAb to either IFN-γ or IL-12 at the time of infection exhibited higher numbers of CFU in lungs and spleens at day 7 of infection. By day 21, all mice administered MAb to either IFN-γ or IL-12 at the time of infection had expired.
TABLE 1.
Recovery of H. capsulatum from lungs and spleens of mice given MAb to IFN-γ and subsequently infecteda
| Treatment | Organ | Mean log10 CFU ± SEM
|
|
|---|---|---|---|
| Day 7 | Day 21 | ||
| Rat IgG for 3 wk prior to infection | Lung | 6.3 ± 0.4 | 3.3 ± 0.2 |
| Spleen | 5.1 ± 0.2 | 2.6 ± 0.3 | |
| MAb to IFN-γ for 3 wk prior to infection | Lung | 6.5 ± 0.6 | 3.5 ± 0.4 |
| Spleen | 5.0 ± 0.3 | 2.4 ± 0.2 | |
| MAb to IFN-γ at the time of infection | Lung | 7.8 ± 0.5b | NDc |
| Spleen | 6.5 ± 0.4b | ND | |
| MAb to IL-12 for 3 wk prior to infection | Lung | 5.9 ± 0.7 | 3.6 ± 0.2 |
| Spleen | 4.6 ± 0.6 | 2.9 ± 0.3 | |
| MAb to IL-12 at the time of infection | Lung | 8.1 ± 0.4b | ND |
| Spleen | 6.3 ± 0.5b | ND | |
Mice (n = 5) were treated with rat IgG, MAb to IFN-γ, or MAb to IL-12 for 3 weeks and then rested for 3 weeks before challenge with 2 × 106 H. capsulatum yeast cells i.n. As a control, a group of mice was given MAb to IFN-γ or MAb to IL-12 at the time of infection. Subsequently, cultures of lungs and spleens were performed at days 7 and 21 postinfection.
P < 0.01 compared to mice treated with rat IgG or MAb to IFN-γ for 3 weeks.
ND, not done. All mice given MAb to IFN-γ or MAb to IL-12 at the time of infection succumbed by day 21.
In the next series of studies, the contribution of these cytokines to the expressive phase of immunity was determined. Mice were vaccinated with rHsp60, and 2 weeks later they were infected with a lethal inoculum of H. capsulatum yeast cells, given MAb to either IFN-γ, IL-10, or IL-12, and monitored for survival. Neutralization of IFN-γ and IL-12, but not IL-10, blocked the expressive phase of protection (Fig. 5B).
We assessed whether the inability of vaccinated mice given MAb to cytokines was associated with an alteration in fungal recovery. The lungs and spleens of mice whose endogenous IFN-γ, IL-10, or IL-12 was neutralized during vaccination contained significantly higher (P < 0.01) levels of CFU than those given rat IgG (Fig. 6A and B). CFU levels in lungs and spleens of mice that received MAb to IFN-γ or IL-12 following vaccination were greater (P < 0.01) than those in animals treated with rat IgG or MAb to IL-10 (Fig. 6C and D).
FIG. 6.
CFU in lungs and spleens of rHsp60-vaccinated mice that were administered MAb to IFN-γ, IL-10, or IL-12. (A and B) Mice (n = 6) were immunized, concomitantly given MAb to cytokines, and challenged 3 weeks later with 1.25 × 107 yeast cells. A group of immunized mice was administered rat IgG. (C and D) For the expressive phase, groups of mice (n = 6) were vaccinated and then administered MAb to cytokines and concomitantly challenged with 1.25 × 107 yeast cells. Immunized controls received rat IgG. CFU were enumerated at day 7 of infection. The data represent mean log10 CFU ± SEM. The results of one of two experiments are shown. #, P < 0.01 compared to immunized controls.
DISCUSSION
Signals delivered between H. capsulatum-sensitized T cells and mononuclear phagocytes direct the outcome of infection with this fungus. The host is often successful in limiting infection when a small number of saprobic forms are inhaled. However, certain at-risk groups may be exposed to a larger quantity of fungal elements, which would jeopardize their health (12). Hence, vaccination is a reasonable approach to either terminate the infection or ameliorate the course of infection among exposed individuals. Moreover, the protection of immunosuppressed individuals from infection as a result of environmental exposure may be best handled by vaccination before the immune system becomes impaired.
Hsp60 from a plethora of microbes is immunoreactive as measured either by a CMI response or by a humoral response (21, 22, 27, 29, 31, 32). The utility of this molecule as a vaccine has been demonstrated for H. capsulatum, Legionella pneumophila, Mycobacterium tuberculosis, and Yersinia enterocolitica (8, 19, 28, 34). Nevertheless, little information exists concerning the mechanism(s) by which this molecule confers protection. To uncover the cellular and molecular determinants that underpin the action of rHsp60, we depleted T-cell subsets or neutralized cytokine activities in mice and examined the capacities of these cytokines to mediate protection as assessed by CFU and by survival. The results demonstrated that the inductive phase of vaccination required the presence of CD4+ cells and IL-12, IL-10, and IFN-γ. Differences emerged when we examined the expressive phase of vaccination. CD4+ or CD8+ cells were dispensable, as was IL-10. The absence of both populations or neutralization of IL-12 or IFN-γ abolished the influence of the vaccine. Since dendritic cells can express CD8 and CD4 (35), it is possible that some of the effect observed following the depletion of CD4+ and CD8+ cells in the expressive phase may have been caused by the loss of this non-T-cell population.
The cytokine responses in the spleens of mice vaccinated with rHsp60 were strikingly different from those in the spleens of mice immunized with rHsp70 or BSA. Hsp70 was selected as an additional control since it has biologic properties similar to those of Hsp60 and it elicits a CMI response in mice, although this response is nonprotective (4, 38). Thus, rHsp70 acts as an excellent foil for the cytokine studies. The data indicate that one of the differences in cytokine release between these two immunogens is a quantitative one. Immunization with rHsp60 elicited more IL-12, IFN-γ, and IL-10 than did an equal amount of rHsp70. The former two are critically important for the generation of a protective immune response both in experimental infection and in vaccination (2, 5, 41). Immunization with rHsp70 did not induce a brisk production of IL-4, a cytokine known to impair the clearance of H. capsulatum (2, 41). One outcome of this study was that we were able to identify one explanation for the greater utility of rHsp60 than of rHsp70 as a protective antigen. The latter is not as potent a stimulus for cytokines that are required for protection as is rHsp60. In addition, Hsp70 does not appear to trigger the production of large quantities of at least one cytokine, IL-4, that has the potential to subvert the expression of a protective immune response. Consequently, the failure of rHsp70 to confer protection is not due to the fact that it induces a cytokine that downregulates the protective immune response.
Hsp60 from both prokaryotes and mammals is a vigorous stimulus of innate immunity as measured by the release of cytokines. Depending on the source of this protein and the target cells, the generation of IL-1β, IL-6, IL-10, IL-12, IL-8 (in humans), GM-CSF, and TNF-α has been reported when mononuclear cells are exposed to Hsp60 directly (9, 23, 25, 30). Immunization with rHsp60 did result in the release of GM-CSF, IL-10, and IL-12 but not TNF-α by spleens. GM-CSF levels were not different than those of rHsp70-immunized cells. IL-1β and IL-6 were not measured, since we limited our analysis to those cytokines that are known to be involved in the protective immune response to this fungus (2, 3, 5, 6, 15, 41). The findings certainly suggest that direct exposure of cells to soluble Hsp60 in solution versus emulsification of it in an adjuvant may generate a similar profile of cytokines by exposed cells.
Both IL-10 and IFN-γ can be released by T cells and non-T cells (16, 26, 33). To explore the source of these two cytokines in mice vaccinated with rHsp60, we depleted CD4+ cells, CD8+ cells, or both and assayed release by antigen-stimulated cells. At each time point, IFN-γ required the presence of CD4+ cells. The only exception was on day 3 after vaccination, when the elimination of CD8+ cells abolished the production of this cytokine. The necessity of CD4+ cells for IFN-γ production by cells from rHsp60-vaccinated mice is similar to that found in experimental histoplasmosis. Elimination of this subpopulation in both primary and secondary pulmonary histoplasmosis abolished IFN-γ production in the lungs of mice (6). Thus, in active infection and in vaccination, CD4+ cells are a major contributor to the release of this vitally important cytokine. The generation of IL-10, on the other hand, was independent of the presence T cells.
The regulatory influence of IL-10 on the inductive and not the expressive phase of vaccination was unanticipated. In experimental histoplasmosis, endogenous IL-10 has only exerted a negative impact on the protective immune response. Mice infected i.n. with H. capsulatum and given MAb to TNF-α or GM-CSF manifest a high mortality in association with elevated levels of IL-10 in lungs (3, 15). In TNF-α-deficient mice, neutralization of IL-4 and IL-10 restores the protective immune response (3), whereas in mice that lack endogenous GM-CSF, blockade of IL-10 can rescue animals (15). IL-10 expresses anti-inflammatory activity (26), but it is doubtful that this property explains its role in vaccine-induced immunity, since the mice were challenged 3 weeks after the cessation of treatment with MAb to IL-10. Conversely, IL-10 can enhance the production of IFN-γ, TNF-α, or GM-CSF when given with IL-12 or IL-18 (10), and exogenous administration of IL-10 augments the circulating levels of IFN-γ (24). It is possible that the positive influence of IL-10 may be a consequence of its capacity to upregulate IFN-γ or other cytokines involved in the protective immune response to this fungus.
The IL-12-IFN-γ axis is a key element in host defenses to primary infection with H. capsulatum yeast cells (2, 41). In mice immunized i.n. with viable yeast cells, IFN-γ, but not IL-12, is necessary for controlling infection (2). These results contrast somewhat with the present findings, in which IL-12 was an essential element in both the inductive and expressive phases of vaccination. Neutralization of IL-12 following vaccination impaired the ability of vaccinated mice to withstand a lethal challenge. The pivotal role of IL-12 in vaccination has been demonstrated in several model systems, but these studies have been limited largely to the inductive phase (1, 20). It is clear that ongoing production of IL-12 during the infection, even after vaccine delivery, is necessary for the maintenance of the protective immune response.
The necessity of IFN-γ mirrors that found in primary and secondary infection in mice (2, 40). These findings extend the importance of this cytokine for fungal vaccination. The efficacy of immunization with the BAD1 (formerly termed WI-1) mutant of the fungus Blastomyces dermatitidis is impaired in mice treated with MAb to IFN-γ (37). Immunization with this mutant, which is hypovirulent, confers protection of mice subsequently exposed to a lethal pulmonary challenge with wild-type yeast cells. Control of the infection in vaccinated mice is dependent on the presence of IFN-γ (37). The pivotal importance of IFN-γ for the success of vaccination appears to be true for both a protein and a replicating attenuated fungal isolate. A major hurdle in vaccination is dealing with those who are immunosuppressed. There are concerns that live attenuated microbes can disseminate in those whose immune systems are defective. One of the critical features of the present findings is that immunized mice whose CD4+ or CD8+ cells were eliminated after vaccination controlled infection. Thus, the loss of important T-cell populations in host defense does not abolish the biological activity of the vaccine (6, 41). The implication of this finding is that the efficacy of vaccination is present even in the absence of either CD4+ or CD8+ cells. Since IFN-γ also was necessary for protection during the expressive phase, the results suggest that each subpopulation of T cells was capable of independently synthesizing this cytokine in quantities that were sufficient for protection. On the other hand, it is possible that another critically important cytokine necessary for protection was synthesized in the absence of either population. Extrapolating to the human condition, these findings suggest that vaccination may protect immunosuppressed individuals if the vaccine is administered when their immune system is intact.
In summary, we have explored the cellular and molecular determinants of immunity in mice vaccinated with rHsp60 from H. capsulatum. The findings can be compared and contrasted with those found for primary and secondary infection with viable yeast cells. The data do not exclude the participation of other cytokines in this complex network but do provide an examination of regulatory elements necessary for vaccine efficacy with this intracellular pathogen.
Acknowledgments
This work was supported by grants AI-34361 and AI-42737 from the National Institute of Allergy and Infectious Diseases and a Merit Review from the Veterans Administration.
Editor: T. R. Kozel
REFERENCES
- 1.Afonso, L. C., T. M. Scharton, L. Q. Vieira, M. Wysocka, G. Trinchieri, and P. Scott. 1994. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science 263:235-237. [DOI] [PubMed] [Google Scholar]
- 2.Allendoerfer, R., G. P. Boivin, and G. S. Deepe, Jr. 1997. Modulation of immune responses in murine pulmonary histoplasmosis. J. Infect. Dis. 175: 905-914. [DOI] [PubMed] [Google Scholar]
- 3.Allendoerfer, R., and G. S. Deepe, Jr. 1998. Blockade of endogenous TNF-α exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J. Immunol. 160:6072-6082. [PubMed] [Google Scholar]
- 4.Allendoerfer, R., B. Maresca, and G. S. Deepe, Jr. 1996. Cellular immune responses to recombinant heat shock protein 70 from Histoplasma capsulatum. Infect. Immun. 64:4123-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allendoerfer, R., and G. S. Deepe, Jr. 1997. Intrapulmonary response to Histoplasma capsulatum in gamma interferon knockout mice. Infect. Immun. 65:2564-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allendörfer-Fernandez, R., G. D. Brunner, and G. S. Deepe, Jr. 1999. Complex requirements for nascent and secondary immunity in pulmonary histoplasmosis. J. Immunol. 162:7389-7396. [PubMed] [Google Scholar]
- 7.Artz, R. P., and W. E. Bullock. 1979. Immunoregulatory responses in experimental disseminated histoplasmosis: depression of T-cell-dependent and T-effector responses by activation of suppressor cells. Infect. Immun. 23:893-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blander, S. J., and M. A. Horowitz. 1993. Major cytoplasmic membrane of Legionella pneumophila, a genus common antigen and member of the Hsp60 family of heat shock proteins, induces protective immunity in a guinea pig model of legionnaires 61 disease. J. Clin. Investig. 91:717-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breloner, M., B. Dorner, B. H. More, T. Roderian, B. Fleisher, and A. von Bonin. 2001. Heat shock proteins as “danger signals”: eukaryotic Hsp60 enhances and accelerates antigen-specific IFN-γ production in T cells. Eur. J. Immunol. 31:2051-2059. [DOI] [PubMed] [Google Scholar]
- 10.Cai, G., R. A. Kastelein, and C. A. Hunter. 1999. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-γ when combined with IL-18. Eur. J. Immunol. 29:2658-2665. [DOI] [PubMed] [Google Scholar]
- 11.Cain, J. A., and G. S. Deepe, Jr. 1998. Evolution of the primary immune response to Histoplasma capsulatum in murine lung. Infect. Immun. 66:1473-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deepe, G. S., Jr. 2000. Histoplasmosis, p. 2718-2734. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practices of infectious diseases, 5th ed., vol. 2. Churchill Livingstone, Philadelphia, Pa. [Google Scholar]
- 13.Deepe, G. S., Jr., and R. Gibbons. 2001. Protective efficacy of H antigen from Histoplasma capsulatum in a murine model of pulmonary histoplasmosis. Infect. Immun. 69:3128-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deepe, G. S., Jr., R. Gibbons, G. D. Brunner, and F. J. Gomez. 1996. A protective domain of heat shock protein 60 from Histoplasma capsulatum. J. Infect. Dis. 174:828-834. [DOI] [PubMed] [Google Scholar]
- 15.Deepe, G. S., Jr., R. Gibbons, and E. Woodward. 1999. Neutralization of endogenous granulocyte-macrophage colony-stimulating factor subverts the protective immune response to Histoplasma capsulatum. J. Immunol. 163:4985-4993. [PubMed] [Google Scholar]
- 16.Frucht, D. M., T. Fukao, C. Bogdan, H. Schindler, J. J. O'Shea, and S. Koyasu. 2001. IFN-γ production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 22:556-560. [DOI] [PubMed] [Google Scholar]
- 17.Gerber, J. S., and D. M. Mosser. 2001. Reversing lipopolysaccharide toxicity by ligating the macrophage Fc gamma receptors. J. Immunol. 166:6861-6868. [DOI] [PubMed] [Google Scholar]
- 18.Gomez, A. M., W. E. Bullock, C. L. Taylor, and G. S. Deepe, Jr. 1988. The role of L3T4+ cells in host defense against Histoplasma capsulatum. Infect. Immun. 56:1685-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez, F. J., R. Allendoerfer, and G. S. Deepe, Jr. 1995. Vaccination with recombinant heat shock protein 60 from Histoplasma capsulatum protects mice against pulmonary histoplasmosis. Infect. Immun. 63:2587-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurunathan, S., C. Prussin, D. L. Sacks, and R. A. Seder. 1998. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 4:1409-1415. [DOI] [PubMed] [Google Scholar]
- 21.Haregewoin, A., G. Soman, R. C. Horn, and R. W. Finberg. 1989. Human γδ+ T cells respond to mycobacterial heat-shock protein. Nature 340:309-312. [DOI] [PubMed] [Google Scholar]
- 22.Izaac, S. M. S., F. J. Gomez, R. S. A. Jesuino, C. A. Fonseca, M. S. S. Felipe, G. S. Deepe, and C. M. A. Soares. 2001. Molecular cloning, characterization and expression the heat shock protein 60 gene from the human pathogenic fungus, Paracoccidoides brasiliensis. Med. Mycol. 39:445-456. [DOI] [PubMed] [Google Scholar]
- 23.Kol, A., A. H. Lichtman, R. W. Finberg, P. Libby, and E. A. Kurt-Jones. 2000. Heat shock protein (hsp) 60 activates the innate immune response: CD14 is an essential receptor for hsp60 activation of mononuclear cells. J. Immunol. 164:13-17. [DOI] [PubMed] [Google Scholar]
- 24.Lauw, F. N., D. Pajkrt, C. E. Hack, M. Kurimoto, S. J. H. van Deventer, and T. van der Poll. 2000. Proinflammatory effects of IL-10 during human endotoxemia. J. Immunol. 165:2783-2789. [DOI] [PubMed] [Google Scholar]
- 25.Lewthwaite, J. C., A. R. M. Coates, P. Tormay, M. Singh, P. Mascagni, S. Poole, M. Roberts, L. Sharp, and B. Henderson. 2001. Mycobacterium tuberculosis chaperonin 60.1 is a more potent cytokine stimulator than chaperonin 60.2 (hsp 65) and contains a CD14-binding domain. Infect. Immun. 69:7349-7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 27.Morrison, R. P., R. J. Belland, K. Lyng, and H. D. Caldwell. 1989. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J. Exp. Med. 170:1271-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noll, A., and I. B. Autenrieth. 1996. Immunity against Yersinia enterocolitica by vaccination with Yersinia Hsp60 immunostimulating complexes or Yersinia Hsp60 plus interleukin-12. Infect. Immun. 64:2955-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noll, A., A. Rogenkamp, J. Heeseman, and I. B. Autenrieth. 1994. Protective role for heat shock protein-reactive alpha/beta T cells in murine yersiniosis. Infect. Immun. 62:2784-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohashi, K., V. Burkart, S. Flohé, and H. Kolb. 2000. Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 164:558-561. [DOI] [PubMed] [Google Scholar]
- 31.Orme, I., P. Andersen, and W. H. Boom. 1993. T cell response to Mycobacterium tuberculosis. J. Infect. Dis. 167: 1481-1497. [DOI] [PubMed] [Google Scholar]
- 32.Rey-Landino, J. A., P. B. Joshi, B. Singh, R. Gupta, and N. E. Reiner. 1997. Leishmania major: molecular cloning, sequencing, and expression of the heat shock protein 60 gene reveals unique carboxy terminal peptide sequences. Exp. Parasitol. 85:249-263. [DOI] [PubMed] [Google Scholar]
- 33.Shibata, Y., L. A. Foster, M. Kurimoto, H. Okamura, R. M. Nakamura, K. Kawajiri, J. P. Justice, M. R. Van Scott, Q. N. Myrvik, and W. J. Metzger. 1998. Immunoregulatory roles of IL-10 in innate immunity: IL-10 inhibits macrophage production of IFN-γ inducing factors but enhances NK cell production of IFN-γ. J. Immunol. 161:4283-4288. [PubMed] [Google Scholar]
- 34.Tascon, R. E., M. J. Colston, S. Ragno, E. Stavropoulos, D. Gregory, and D. B. Lowrie. 1996. Vaccination against tuberculosis by DNA injection. Nat. Med. 2:888-892. [DOI] [PubMed] [Google Scholar]
- 35.Vremec, D., J. Pooley, H. Hochrein, L. Wu, and K. Shortman. 2000. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 164:2978-2986. [DOI] [PubMed] [Google Scholar]
- 36.Wu-Hsieh, B. A., and D. H. Howard. 1984. Inhibition of growth of Histoplasma capsulatum by lymphokine-stimulated macrophages. J. Immunol. 132:2593-2597. [PubMed] [Google Scholar]
- 37.Wüthrich, M., H. I. Filutowicz, and B. S. Klein. 2000. Mutation of the WI-1 gene yields an attenuated Blastomyces dermatitidis strain that induces host resistance. J. Clin. Investig. 106:1381-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young, R. A., and T. J. Elliot. 1989. Stress proteins, infection, and immune surveillance. Cell 59:5-8. [DOI] [PubMed] [Google Scholar]
- 39.Zhou, P., and R. A. Seder. 1998. CD40 ligand is not essential for induction of type 1 cytokine responses or protective immunity after primary or secondary infection with Histoplasma capsulatum. J. Exp. Med. 187:1315-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou, P., G. Miller, and R. A. Seder. 1998. Factors involved in regulating primary and secondary immunity to infection with Histoplasma capsulatum: TNF-α plays a critical role in maintaining secondary immunity in the absence of IFN-γ. J. Immunol. 160:1359-1368. [PubMed] [Google Scholar]
- 41.Zhou, P., M. C. Sieve, J. Bennett, J. Kwon-Chung, R. P. Tewari, R. T. Gazinelli, A. Sher, and R. A. Seder. 1995. IL-12 prevents mortality in mice infected Histoplasma capsulatum through induction of IFN-γ. J. Immunol. 155:785-795. [PubMed] [Google Scholar]