Abstract
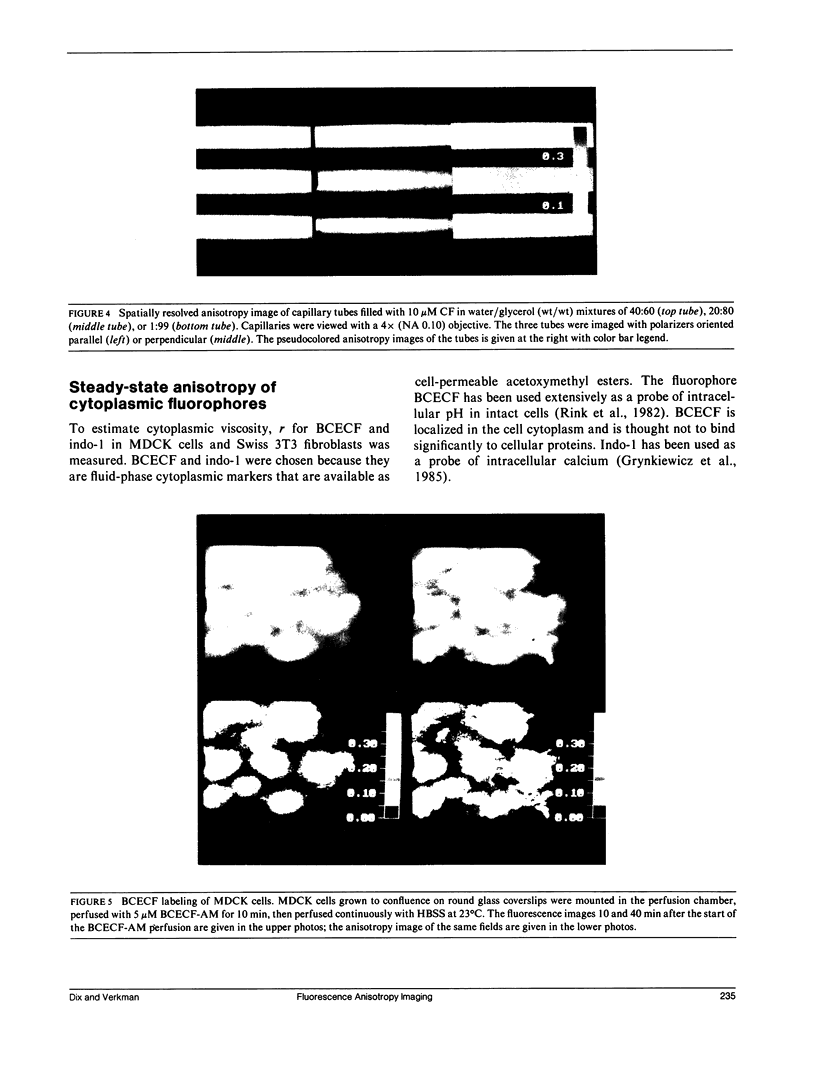
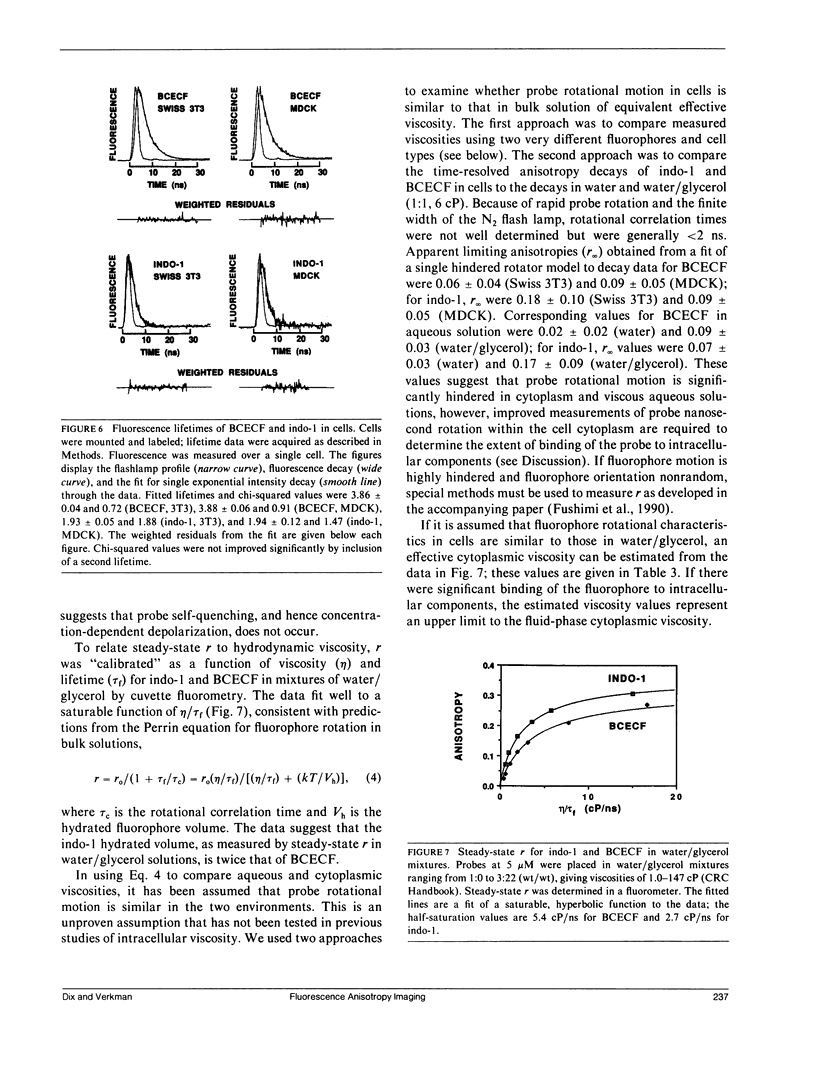
Steady-state and time-resolved fluorescence properties of probes incorporated into living cells give information about the microenvironment near the probe. We have extended studies of spatially averaged fluorescence anisotropy (r) by using an epifluorescence microscope, equipped with excitation and emission polarizers and an image analysis system, to map r of nonoriented fluorophores incorporated into cultured cells. With this imaging system, r for reflected light or glycogen scattering solutions was greater than 0.98. Measurement of r over the range 0.01-0.35 for fluorophores in bulk solution and in thin capillary tubes placed side-by-side gave values equivalent to r measured by cuvette fluorometry. Cytoplasmic viscosity (eta) in Madin-Darby canine kidney (MDCK) cells and Swiss 3T3 fibroblasts was examined from anisotropy images and time-resolved fluorescence decay of the cytoplasmic probes 2,7-bis-carboxyethyl-5 (and 6)-carboxy-fluorescein (BCECF) and indo-1. Nanosecond lifetimes and anisotropy decay were measured using a pulsed light source and gated detector interfaced to the epifluorescence microscope. Anisotropy images of BCECF in MDCK cells revealed two distinct regions of r: one from the cytoplasm (r = 0.144 +/- 0.008) and a second appearing at late times from the interstitial region (r = 0.08 +/- 0.03), representing BCECF trapped beneath the tight junctions. Anisotropy values, taken together with intracellular life-times and the calibration between r and eta/tau f for water/glycerol mixtures, gave eta values of 10-13 cP at 23 degrees C. These values assume little fluorophore binding to intracellular components and are therefore upper limits to cytoplasmic viscosity. These data establish a new methodology to map anisotropy in intact cells to examine the role of fluidity in cellular physiology.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D. Carbocyanine dye orientation in red cell membrane studied by microscopic fluorescence polarization. Biophys J. 1979 Jun;26(3):557–573. doi: 10.1016/S0006-3495(79)85271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D. Fluorescence polarization microscopy. Methods Cell Biol. 1989;30:333–352. [PubMed] [Google Scholar]
- Burns V. W. Measurement of viscosity in living cells by a fluorescence method. Biochem Biophys Res Commun. 1969 Dec 4;37(6):1008–1014. doi: 10.1016/0006-291x(69)90232-0. [DOI] [PubMed] [Google Scholar]
- Chao A. C., Dix J. A., Sellers M. C., Verkman A. S. Fluorescence measurement of chloride transport in monolayer cultured cells. Mechanisms of chloride transport in fibroblasts. Biophys J. 1989 Dec;56(6):1071–1081. doi: 10.1016/S0006-3495(89)82755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushimi K., Dix J. A., Verkman A. S. Cell membrane fluidity in the intact kidney proximal tubule measured by orientation-independent fluorescence anisotropy imaging. Biophys J. 1990 Feb;57(2):241–254. doi: 10.1016/S0006-3495(90)82527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb D., Nord E. P. Asymmetric affinity of Na+-H+ antiporter for Na+ at the cytoplasmic versus external transport site. Am J Physiol. 1987 Nov;253(5 Pt 2):F959–F968. doi: 10.1152/ajprenal.1987.253.5.F959. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hashimoto Y., Shinozaki N. Measurement of cytoplasmic viscosity by fluorescence polarization in phytohemagglutinin-stimulated and unstimulated human peripheral lymphocytes. J Histochem Cytochem. 1988 Jun;36(6):609–613. doi: 10.1177/36.6.3367046. [DOI] [PubMed] [Google Scholar]
- Hise M. K., Mantulin W. W., Weinman E. J. Fluidity and composition of brush border and basolateral membranes from rat kidney. Am J Physiol. 1984 Sep;247(3 Pt 2):F434–F439. doi: 10.1152/ajprenal.1984.247.3.F434. [DOI] [PubMed] [Google Scholar]
- Illsley N. P., Lin H. Y., Verkman A. S. Lipid domain structure correlated with membrane protein function in placental microvillus vesicles. Biochemistry. 1987 Jan 27;26(2):446–454. doi: 10.1021/bi00376a016. [DOI] [PubMed] [Google Scholar]
- Illsley N. P., Verkman A. S. Membrane chloride transport measured using a chloride-sensitive fluorescent probe. Biochemistry. 1987 Mar 10;26(5):1215–1219. doi: 10.1021/bi00379a002. [DOI] [PubMed] [Google Scholar]
- Krapf R., Illsley N. P., Tseng H. C., Verkman A. S. Structure-activity relationships of chloride-sensitive fluorescent indicators for biological application. Anal Biochem. 1988 Feb 15;169(1):142–150. doi: 10.1016/0003-2697(88)90265-5. [DOI] [PubMed] [Google Scholar]
- Le Grimellec C., Giocondi M. C., Carrière B., Carrière S., Cardinal J. Membrane fluidity and enzyme activities in brush border and basolateral membranes of the dog kidney. Am J Physiol. 1982 Mar;242(3):F246–F253. doi: 10.1152/ajprenal.1982.242.3.F246. [DOI] [PubMed] [Google Scholar]
- Lepock J. R., Cheng K. H., Campbell S. D., Kruuv J. Rotational diffusion of TEMPONE in the cytoplasm of Chinese hamster lung cells. Biophys J. 1983 Dec;44(3):405–412. doi: 10.1016/S0006-3495(83)84314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmo T., Steen H. B. Flow cytometric measurement of the polarization of fluorescence from intracellular fluorescein in mammalian cells. Biophys J. 1977 May;18(2):173–187. doi: 10.1016/S0006-3495(77)85606-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K., Lanni F., Taylor D. L. The submicroscopic properties of cytoplasm as a determinant of cellular function. Annu Rev Biophys Biophys Chem. 1988;17:369–396. doi: 10.1146/annurev.bb.17.060188.002101. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K., Taylor D. L., Lanni F. Probing the structure of cytoplasm. J Cell Biol. 1986 Jun;102(6):2015–2022. doi: 10.1083/jcb.102.6.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luly P., Shinitzky M. Gross structural changes in isolated liver cell plasma membranes upon binding of insulin. Biochemistry. 1979 Feb 6;18(3):445–450. doi: 10.1021/bi00570a009. [DOI] [PubMed] [Google Scholar]
- Mastro A. M., Babich M. A., Taylor W. D., Keith A. D. Diffusion of a small molecule in the cytoplasm of mammalian cells. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3414–3418. doi: 10.1073/pnas.81.11.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K. R. The cytomatrix: a short history of its study. J Cell Biol. 1984 Jul;99(1 Pt 2):3s–12s. doi: 10.1083/jcb.99.1.3s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaggio A. M., Schwartz J. H., Bengele H. H., Alexander E. A. Kinetics of the Na+-H+ antiporter as assessed by the change in intracellular pH in MDCK cells. Am J Physiol. 1986 Oct;251(4 Pt 1):C558–C562. doi: 10.1152/ajpcell.1986.251.4.C558. [DOI] [PubMed] [Google Scholar]
- Verkman A. S., Ives H. E. Water permeability and fluidity of renal basolateral membranes. Am J Physiol. 1986 Apr;250(4 Pt 2):F633–F643. doi: 10.1152/ajprenal.1986.250.4.F633. [DOI] [PubMed] [Google Scholar]
- Verkman A. S., Masur S. K. Very low osmotic water permeability and membrane fluidity in isolated toad bladder granules. J Membr Biol. 1988 Sep;104(3):241–251. doi: 10.1007/BF01872326. [DOI] [PubMed] [Google Scholar]