Abstract
The regulation of the cell-to-cell pathway formed by gap junctions seems to involve the interaction of the junctional channels with either calcium or hydrogen ions, as well as protein phosphorylation and calmodulin. These mechanisms of junctional regulation have been considered to act independently on specific sites of the gap junction protein; however, the possibility that they may be interrelated has not been adequately explored mainly due to the difficulties involved in simultaneous measurement of intracellular cations and protein phosphorylation. To further understanding of mechanisms regulating gap junctions, we have internally perfused coupled lateral axons from crayfish with solutions containing different calcium and hydrogen concentrations under conditions favoring phosphorylation, while monitoring the junctional conductance. We found that calcium ions regulate cell communication probably through a direct interaction with the channel protein. Phosphorylation and low pH do not alter junctional conductance themselves, but appear only to modulate the effects of calcium, possibly by altering the affinity of the channel for calcium. We propose that a combination of free intracellular calcium and protein phosphorylation form an important physiological mechanism regulating intercellular communication.
Full text
PDF

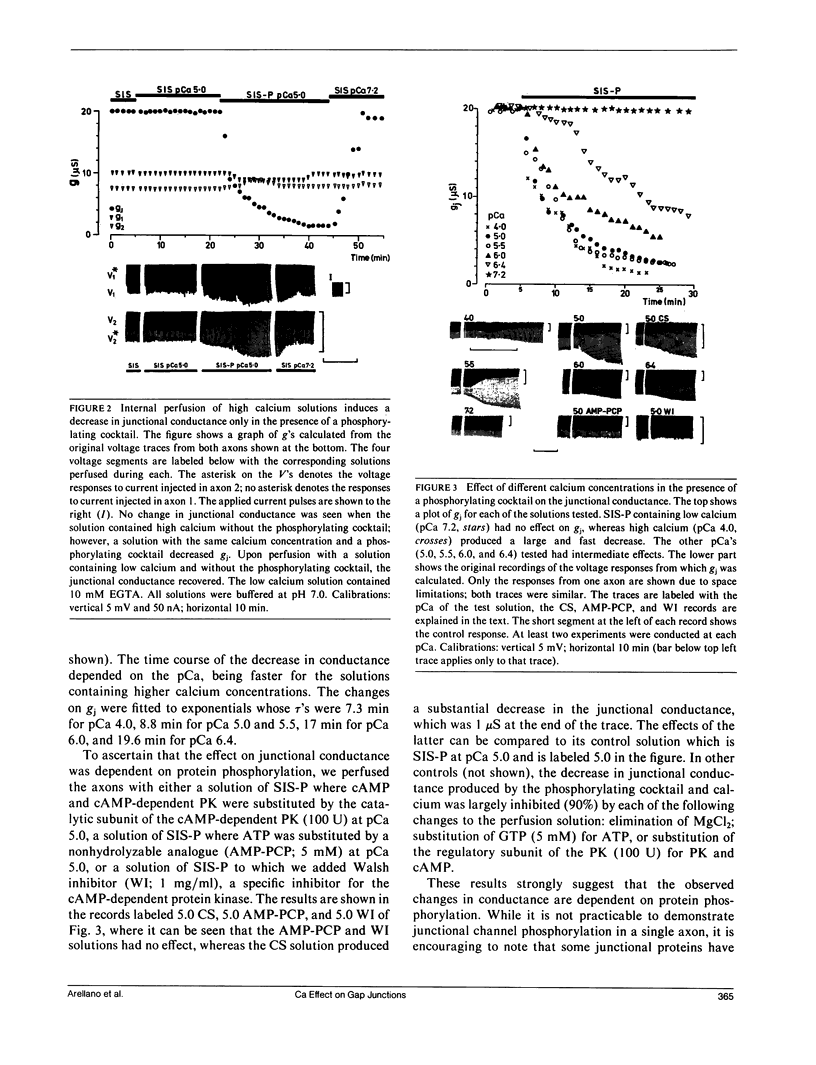
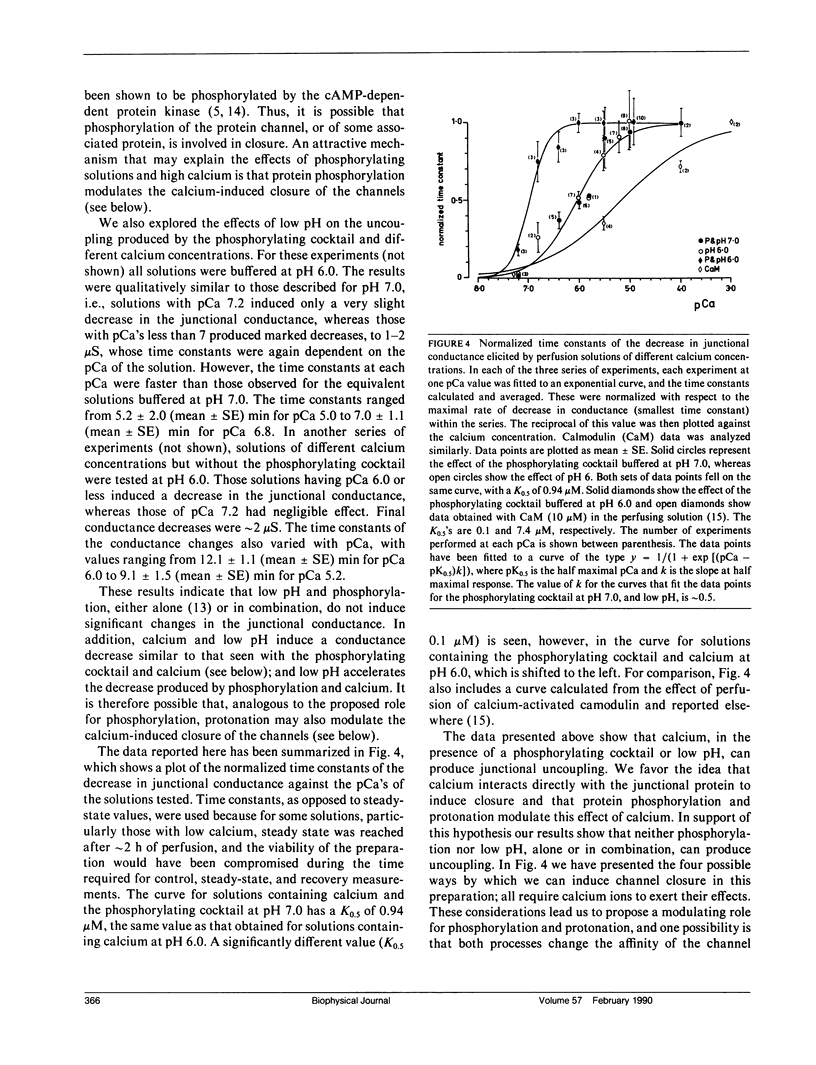

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arellano R. O., Ramón F., Rivera A., Zampighi G. A. Calmodulin acts as an intermediary for the effects of calcium on gap junctions from crayfish lateral axons. J Membr Biol. 1988;101(2):119–131. doi: 10.1007/BF01872827. [DOI] [PubMed] [Google Scholar]
- Arellano R. O., Ramón F., Rivera A., Zampighi G. A. Lowering of pH does not directly affect the junctional resistance of crayfish lateral axons. J Membr Biol. 1986;94(3):293–299. doi: 10.1007/BF01869725. [DOI] [PubMed] [Google Scholar]
- Azarnia R., Reddy S., Kmiecik T. E., Shalloway D., Loewenstein W. R. The cellular src gene product regulates junctional cell-to-cell communication. Science. 1988 Jan 22;239(4838):398–401. doi: 10.1126/science.2447651. [DOI] [PubMed] [Google Scholar]
- Johnston M. F., Ramón F. Electrotonic coupling in internally perfused crayfish segmented axons. J Physiol. 1981 Aug;317:509–518. doi: 10.1113/jphysiol.1981.sp013840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M. Retinal horizontal cell gap junctional conductance is modulated by dopamine through a cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7319–7323. doi: 10.1073/pnas.84.20.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein W. R. Permeability of membrane junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):441–472. doi: 10.1111/j.1749-6632.1966.tb50175.x. [DOI] [PubMed] [Google Scholar]
- Noma A., Tsuboi N. Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. J Physiol. 1987 Jan;382:193–211. doi: 10.1113/jphysiol.1987.sp016363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracchia C., Bernardini G., Peracchia L. L. Is calmodulin involved in the regulation of gap junction permeability? Pflugers Arch. 1983 Oct;399(2):152–154. doi: 10.1007/BF00663912. [DOI] [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H. M. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984 Oct;4(10):2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón F., Rivera A. Gap junction channel modulation--a physiological viewpoint. Prog Biophys Mol Biol. 1986;48(3):127–153. doi: 10.1016/0079-6107(86)90010-6. [DOI] [PubMed] [Google Scholar]
- Ramón F., Zampighi G. On the electrotonic coupling mechanism of crayfish segmented axons: temperature dependence of junctional conductance. J Membr Biol. 1980 Jun 15;54(3):165–171. doi: 10.1007/BF01870232. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Saez J. C., Spray D. C., Nairn A. C., Hertzberg E., Greengard P., Bennett M. V. cAMP increases junctional conductance and stimulates phosphorylation of the 27-kDa principal gap junction polypeptide. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2473–2477. doi: 10.1073/pnas.83.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981 Feb 13;211(4483):712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Takeda A., Hashimoto E., Yamamura H., Shimazu T. Phosphorylation of liver gap junction protein by protein kinase C. FEBS Lett. 1987 Jan 5;210(2):169–172. doi: 10.1016/0014-5793(87)81330-3. [DOI] [PubMed] [Google Scholar]
- Turin L., Warner A. Carbon dioxide reversibly abolishes ionic communication between cells of early amphibian embryo. Nature. 1977 Nov 3;270(5632):56–57. doi: 10.1038/270056a0. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Ennis P. D. Calcium-mediated changes in gap junction structure: evidence from the low angle X-ray pattern. J Cell Biol. 1983 Nov;97(5 Pt 1):1459–1466. doi: 10.1083/jcb.97.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi G., Kreman M., Ramón F., Moreno A. L., Simon S. A. Structural characteristics of gap junctions. I. Channel number in coupled and uncoupled conditions. J Cell Biol. 1988 May;106(5):1667–1678. doi: 10.1083/jcb.106.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]



