Abstract
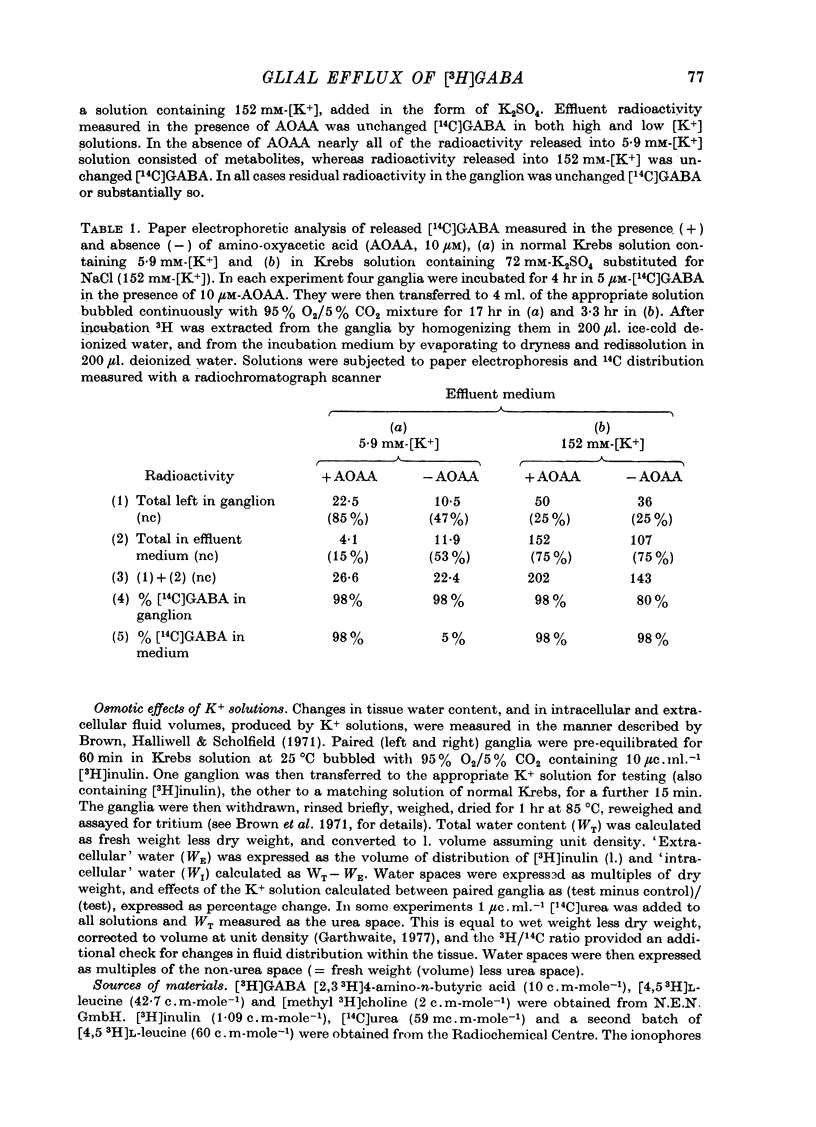
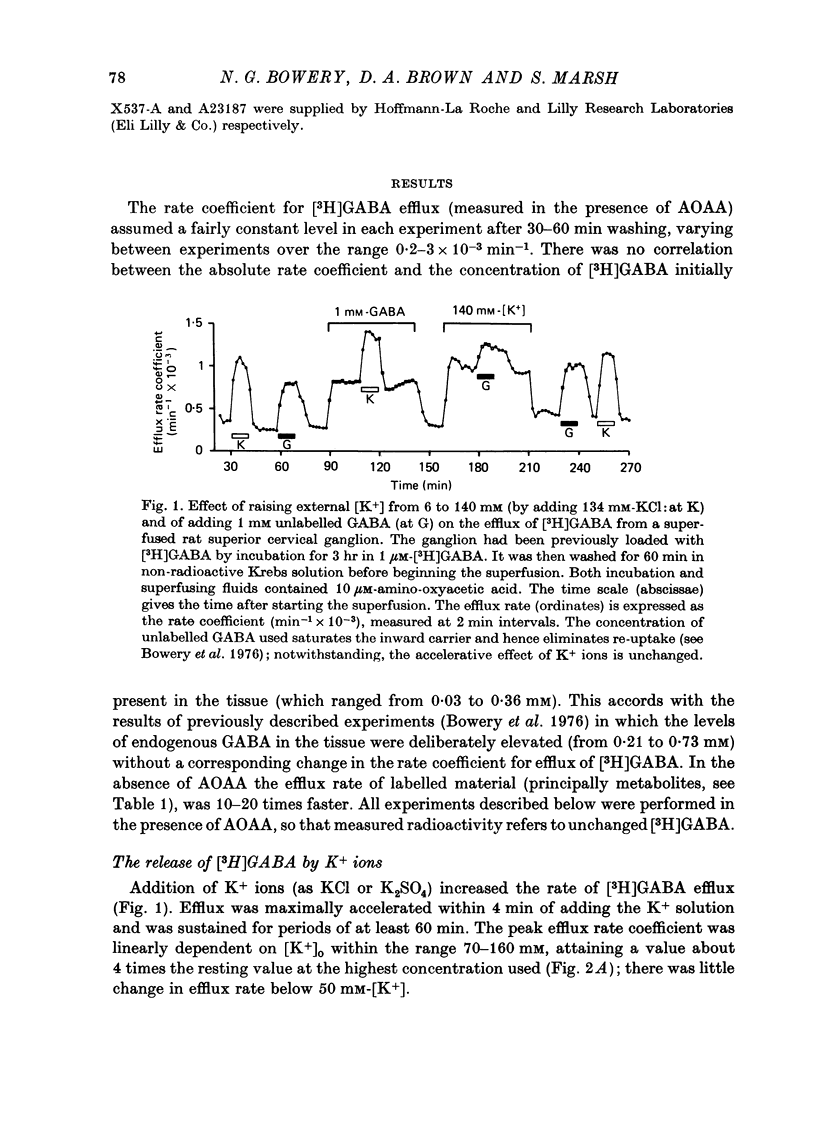
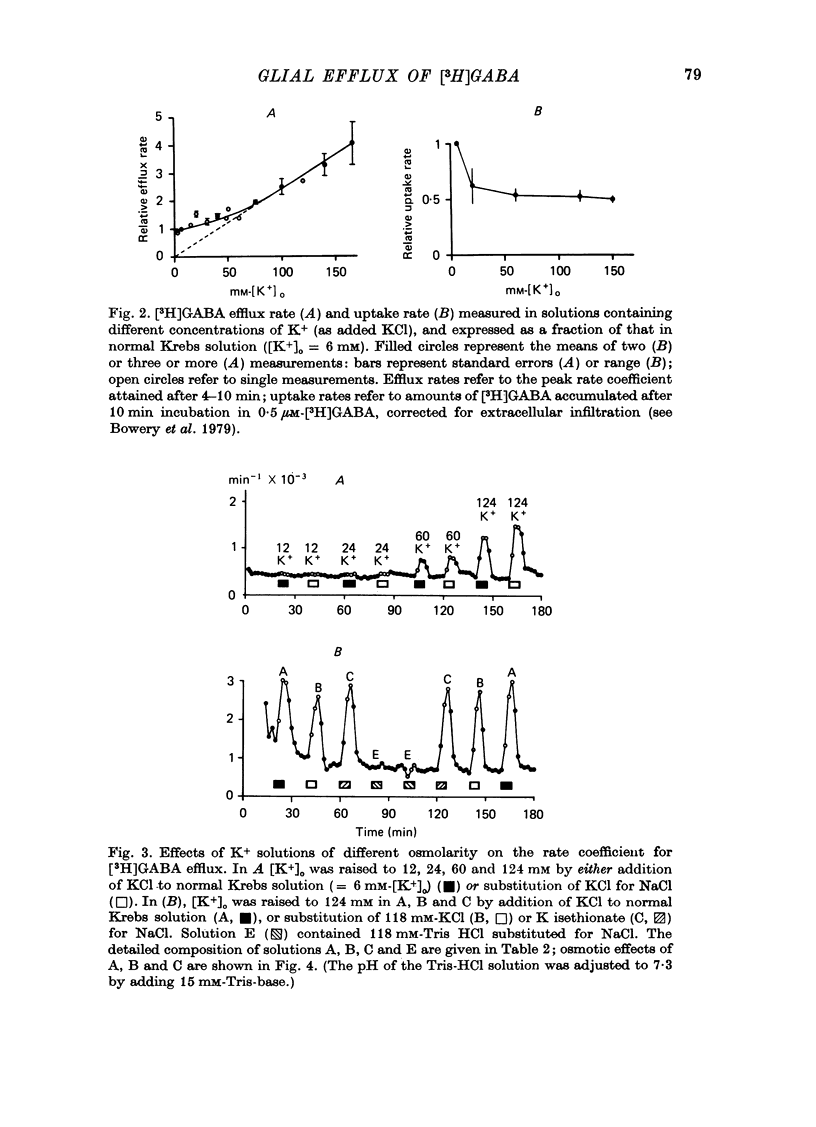
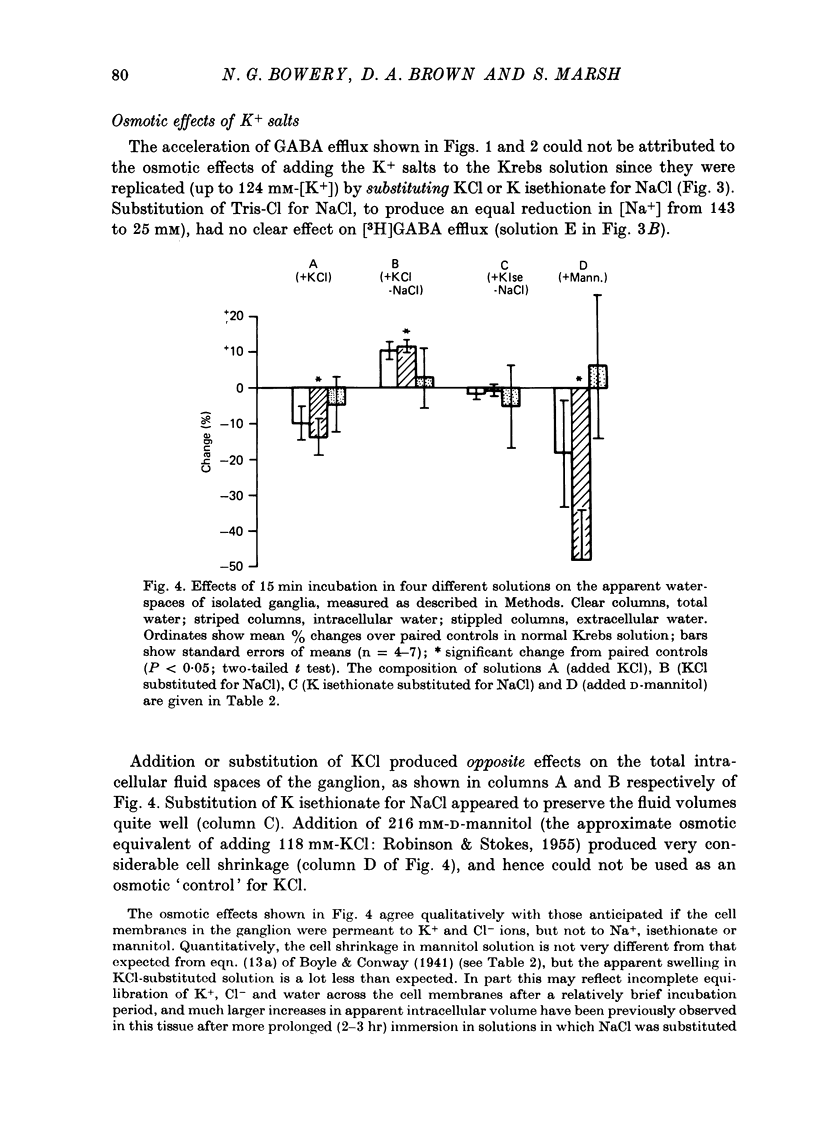
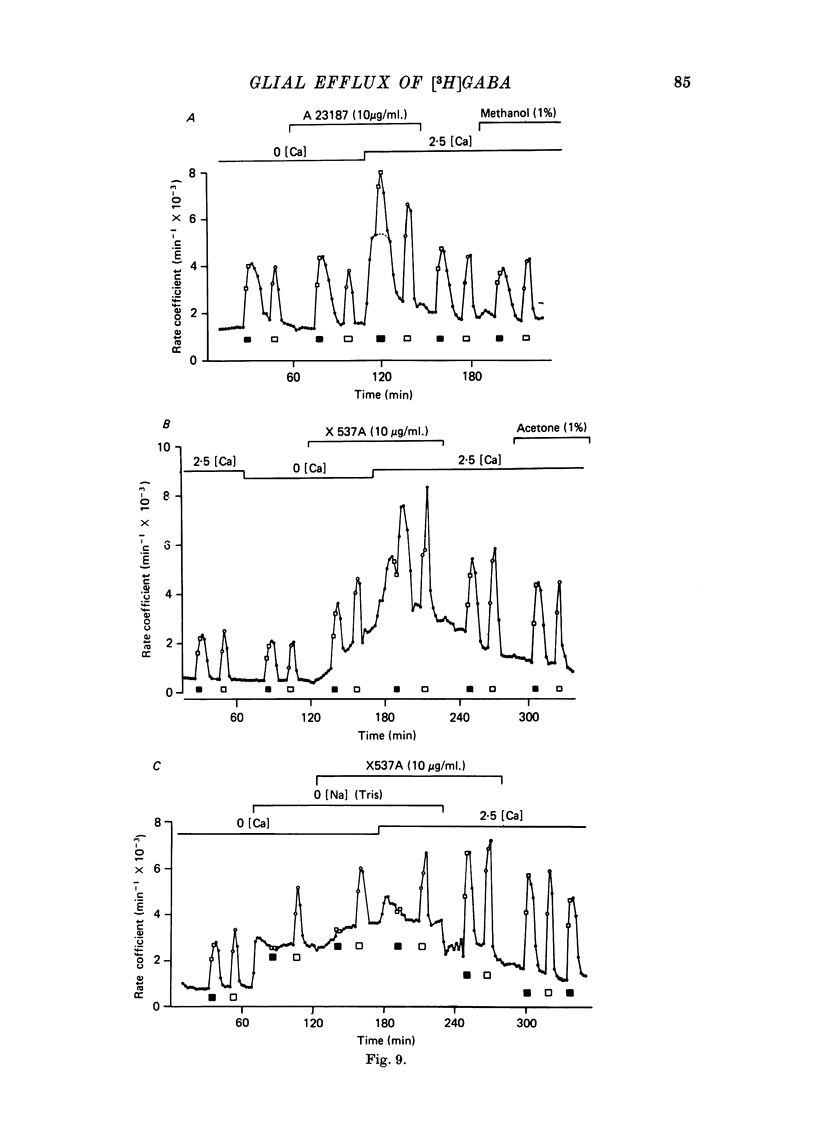
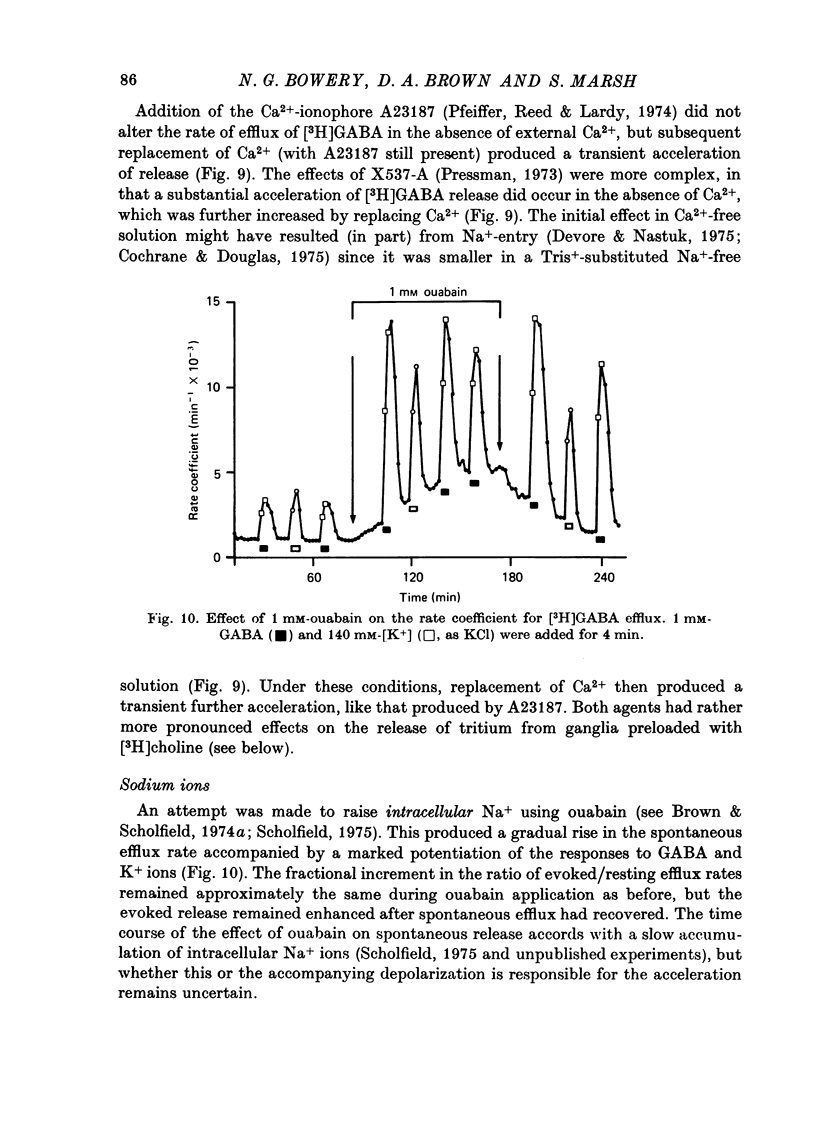
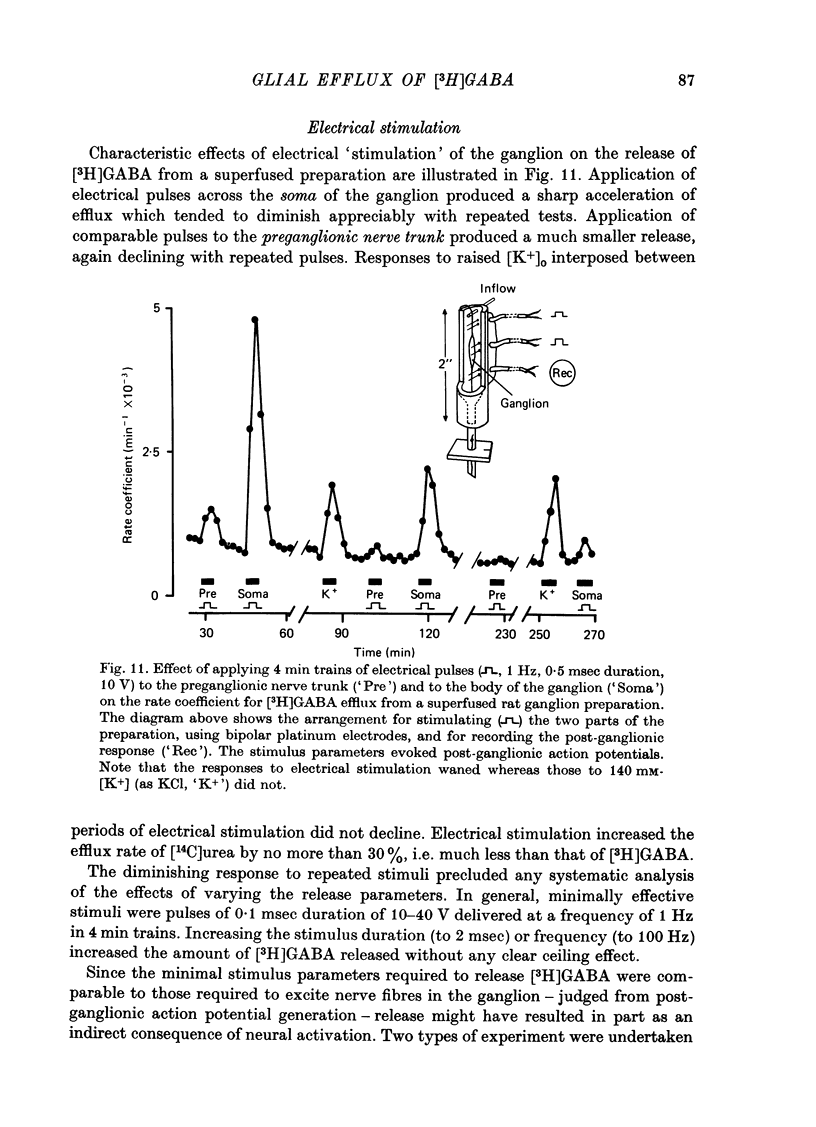
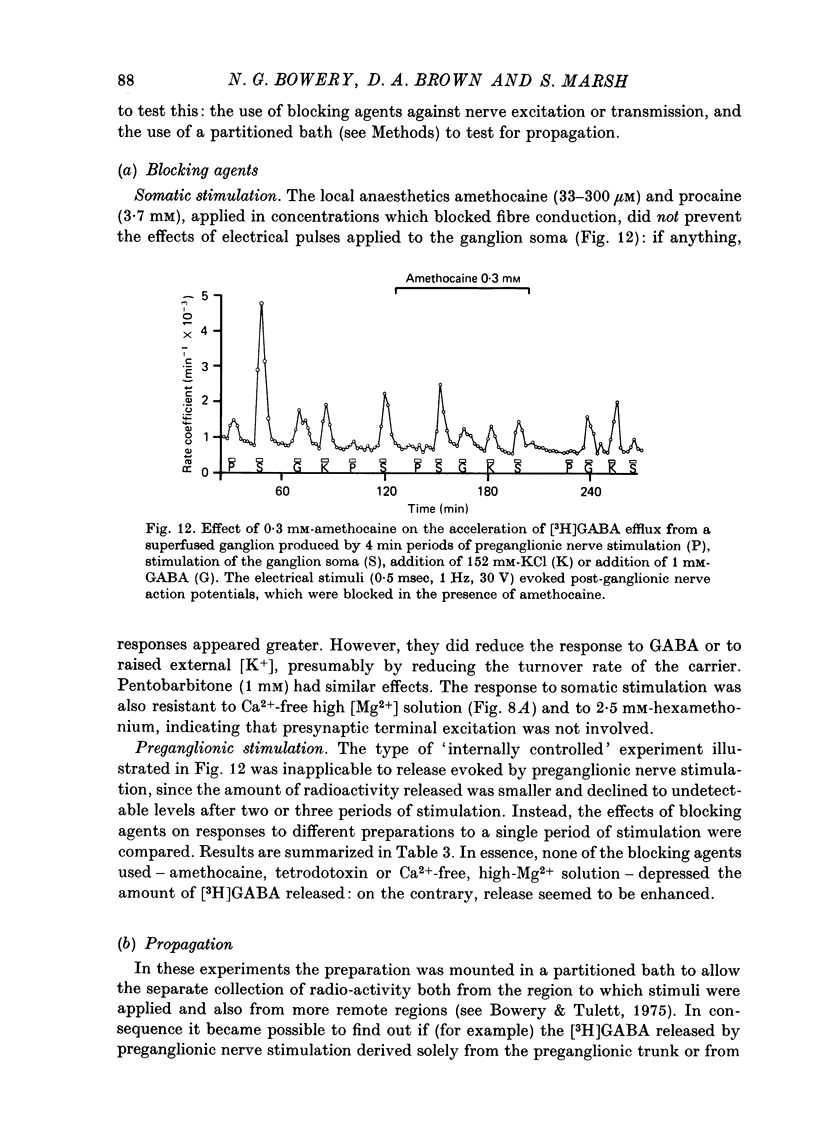
1. Isolated desheathed rat superior cervical ganglia were incubated in [3H]2,3,-gamma-aminobutyric acid ([3H]GABA) solution (1--10 microM for 2--3 hr) in the presence of 10 microM-amino-oxyacetic acid (AOAA). The subsequent efflux of tritium into a stream of superfused non-radioactive GABA-free Krebs solution at 25 degrees C was measured. 2. In the presence of 10 micrometer-AOAA the mean basal efflux rate coefficient (k0) for exit of tritium into the superfusion fluid was 0.7 x 10(-3) min-1. More than 98% of effluent tritium comprised unchanged [3H]GABA. The rate coefficient showed no correlation with the amount of [3H]GABA previously accumulated by the ganglion. 3. Elevation of [K+]o to greater than 50 mM increased the rate coefficient for [3H]GABA release by up to four times. Changes in efflux rate were not correlated with osmotic changes, and persisted after re-accumulation of effluent [3H]GABA by the inward carrier was inhibited. The effect of alkali metal cations diminished in the order Rb+ greater than K+ greater than Cs+Li+. Effects of K+ solutions were not reduced by omitting Ca2+ ions, with or without the addition of Mg2+. 4. Application of electrical pulses (0.1--1 msec duration, 1--10 Hz, 4 min trains) to the ganglion soma or to the preganglionic nerve trunk also raised k0. This effect declined with repeated stimulus trains, without an accompanying diminution in the response to K+. Responses to electrical stimulation were not reduced by amethocaine (300 microM), tetrodotoxin (3 microM) or raised [Mg2+i1 (0 mM-[Ca2+]/30 mM-[Mg3+]). Separate local superfusion of the pre- and post-ganglionic nerve trunks and of the ganglion soma showed that the response to electrical stimulation was localized to the vicinity of the stimulus and was not propagated along the nerve trunks or across the synapses. 5. Electrical recording from impaled 'inexcitable' cells (presumed to be neuroglial cells (Appendix)) indicated that the quantities of K+ ion accumulating during repetitive nerve stimulation are insufficient to stimulate the release of GABA from the glial cells. No physiological role for the release process in modulating neuronal excitability could be adduced.
Full text
PDF

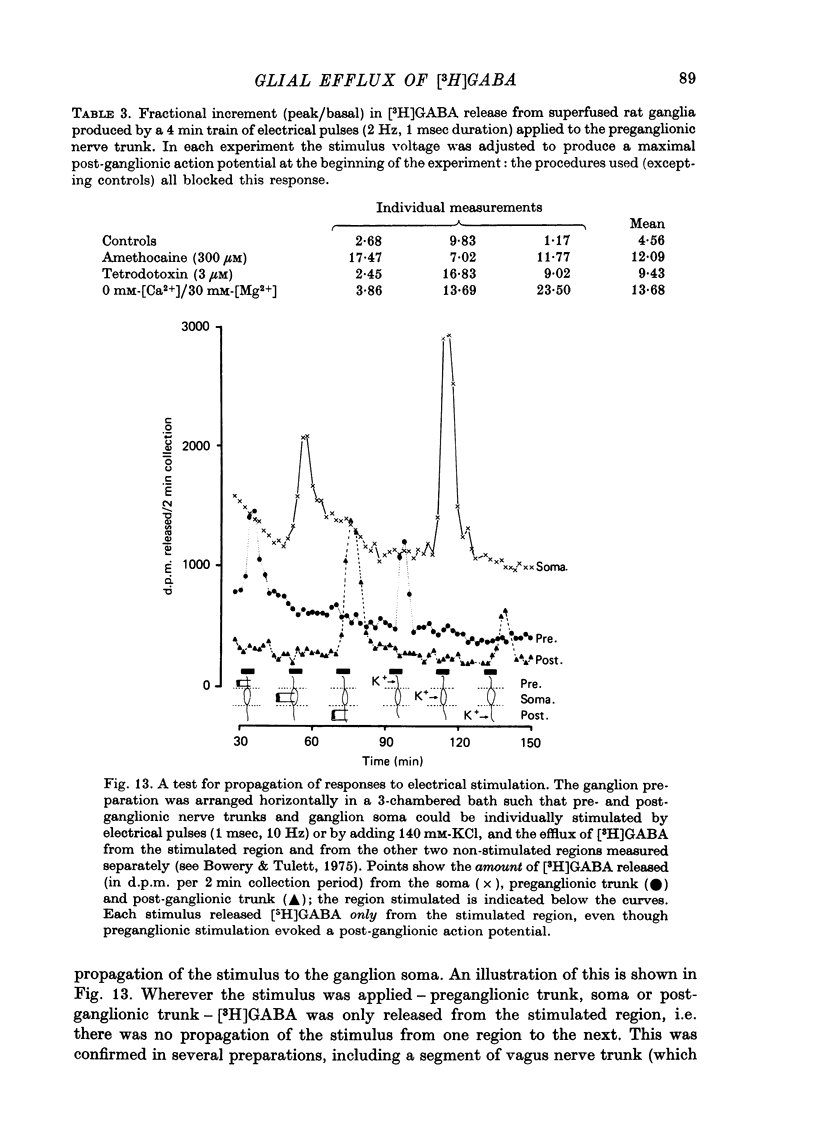
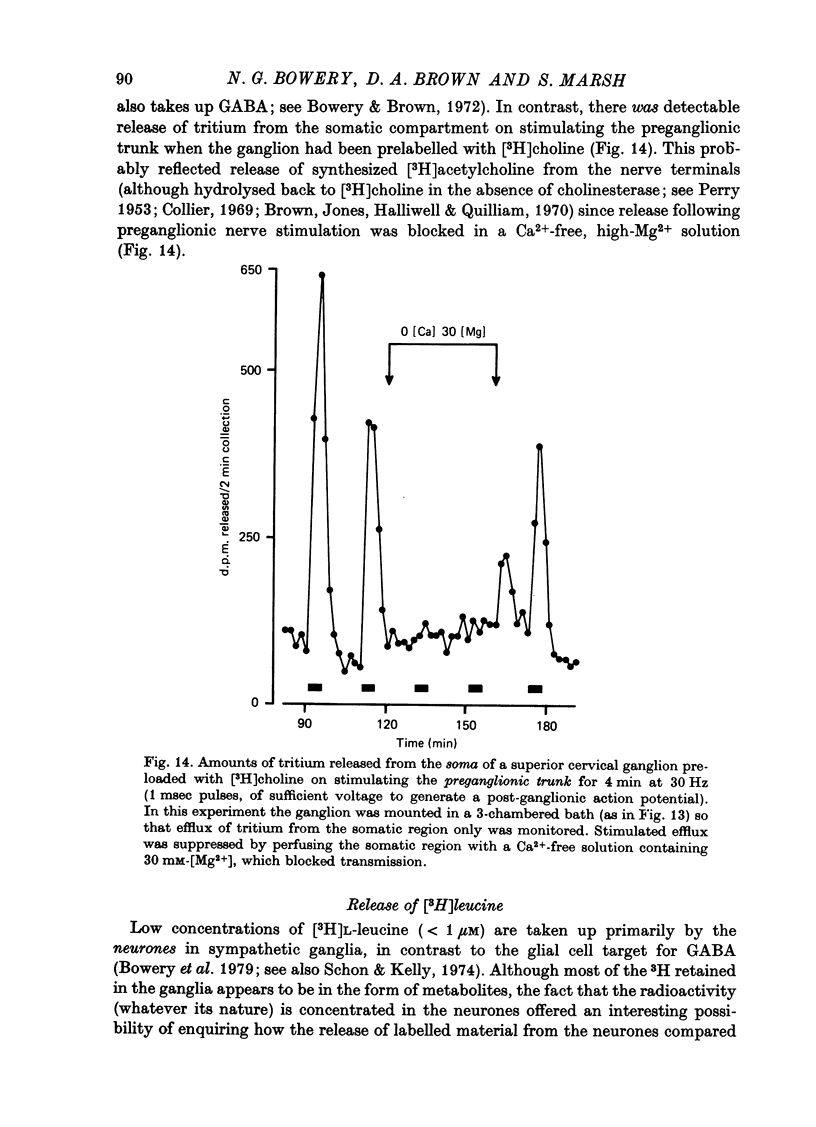
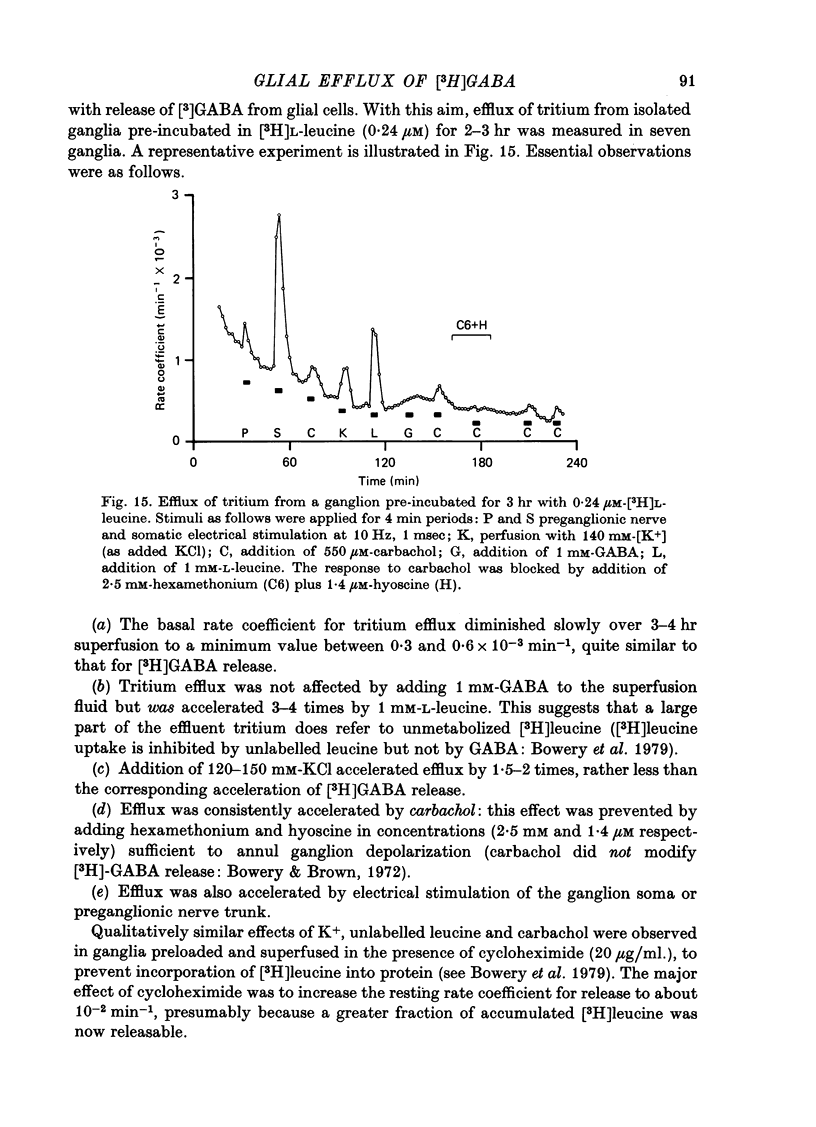




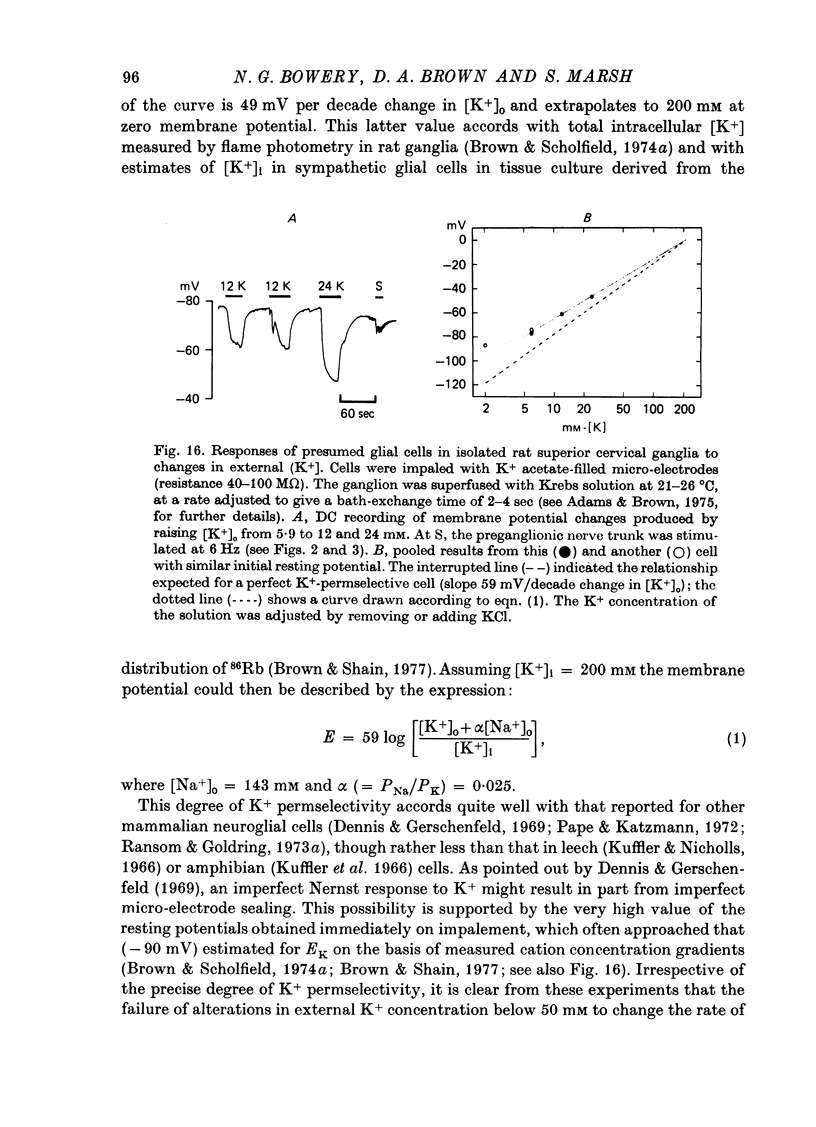




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair R., Davidoff R. A. Studies of the uptake and release of [3H]beta-alanine by frog spinal slices. J Neurochem. 1977 Aug;29(2):213–220. doi: 10.1111/j.1471-4159.1977.tb09611.x. [DOI] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Crowcroft P. J., Devine C. E., Holman M. E., Yonemura K. Transmission from pregnanglionic fibres in the hypogastric nerve to peripheral ganglia of male guinea-pigs. J Physiol. 1969 May;201(3):723–743. doi: 10.1113/jphysiol.1969.sp008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Purves R. D. Intracellular recordings from ganglia of the thoracic sympathetic chain of the guinea-pig. J Physiol. 1969 Jul;203(1):173–198. doi: 10.1113/jphysiol.1969.sp008858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., King A. C. Influence of membrane potential on the sodium-dependent uptake of gamma-aminobutyric acid by presynaptic nerve terminals: experimental observations and theoretical considerations. J Membr Biol. 1976 Dec 28;30(2):153–173. doi: 10.1007/BF01869665. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A. -Aminobutyric acid uptake by sympathetic ganglia. Nat New Biol. 1972 Jul 19;238(81):89–91. doi: 10.1038/newbio238089a0. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A., Collins G. G., Galvan M., Marsh S., Yamini G. Indirect effects of amino-acids on sympathetic ganglion cells mediated through the release of gamma-aminobutyric acid from glial cells. Br J Pharmacol. 1976 May;57(1):73–91. doi: 10.1111/j.1476-5381.1976.tb07658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A. Depolarizing actions of gamma-aminobutyric acid and related compounds on rat superior cervical ganglia in vitro. Br J Pharmacol. 1974 Feb;50(2):205–218. doi: 10.1111/j.1476-5381.1974.tb08563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A. Proceedings: On the release of accumulated (3-H)-gamma-aminobutyric acid (GABA) from isolated rat superior cervical ganglia. Br J Pharmacol. 1974 Nov;52(3):436P–437P. [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A., White R. D., Yamini G. [3H]gamma-Aminobutyric acid uptake into neuroglial cells of rat superior cervical sympathetic ganglia. J Physiol. 1979 Aug;293:51–74. doi: 10.1113/jphysiol.1979.sp012878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford H. F. Metabolic response of synaptosomes to electrical stimulation: release of amino acids. Brain Res. 1970 Apr 14;19(2):239–247. doi: 10.1016/0006-8993(70)90437-3. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Brownstein M. J., Scholfield C. N. Origin of the after-hyperpolarization that follows removal of depolarizing agents from the isolated superior cervical ganglion of the rat. Br J Pharmacol. 1972 Apr;44(4):651–671. doi: 10.1111/j.1476-5381.1972.tb07305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Halliwell J. V. Intracellular pH in rat isolated superior cervical ganglia in relation to nicotine-depolarization and nicotine-uptake. Br J Pharmacol. 1972 Jun;45(2):349–359. doi: 10.1111/j.1476-5381.1972.tb08088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Halliwell J. V., Scholfield C. N. Uptake of nicotine and extracellular space markers by isolated rat ganglia in relation to receptor activation. Br J Pharmacol. 1971 May;42(1):100–113. doi: 10.1111/j.1476-5381.1971.tb07090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Jones K. B., Halliwell J. V., Quilliam J. P. Evidence against a presynaptic action of acetylcholine during ganglionic transmission. Nature. 1970 Jun 6;226(5249):958–959. doi: 10.1038/226958a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Marsh S. Axonal GABA-receptors in mammalian peripheral nerve trunks. Brain Res. 1978 Nov 3;156(1):187–191. doi: 10.1016/0006-8993(78)90098-7. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Changes of intracellular sodium and potassium ion concentrations in isolated rat superior cervical ganglia induced by depolarizing agents. J Physiol. 1974 Oct;242(2):307–319. doi: 10.1113/jphysiol.1974.sp010709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Movements of labelled sodium ions in isolated rat superior cervical ganglia. J Physiol. 1974 Oct;242(2):321–351. doi: 10.1113/jphysiol.1974.sp010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Nicotine washout rates from isolated rat ganglia in relation to recovery from nicotine depolarization. Br J Pharmacol. 1972 May;45(1):29–36. doi: 10.1111/j.1476-5381.1972.tb09573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Shain W. Cation concentration gradients in cultured sympathetic neuroglial cells [proceedings]. J Physiol. 1977 Jul;269(1):45P–46P. [PubMed] [Google Scholar]
- Cohrane D. E., Douglas W. W. Depolarizing effects of the ionophores X-537A and A23187 and their relevance to secretion. Br J Pharmacol. 1975 Jul;54(3):400–402. doi: 10.1111/j.1476-5381.1975.tb07582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier B. The preferential release of newly synthesized transmitter by a sympathetic ganglion. J Physiol. 1969 Nov;205(2):341–352. doi: 10.1113/jphysiol.1969.sp008969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Belleroche J. S., Bradford H. F. Metabolism of beds of mammalian cortical synaptosomes: response to depolarizing influences. J Neurochem. 1972 Mar;19(3):585–602. doi: 10.1111/j.1471-4159.1972.tb01376.x. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Gerschenfeld H. M. Some physiological properties of identified mammalian neuroglial cells. J Physiol. 1969 Jul;203(1):211–222. doi: 10.1113/jphysiol.1969.sp008860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Electrically induced release of acetylcholine from denervated Schwann cells. J Physiol. 1974 Mar;237(2):431–452. doi: 10.1113/jphysiol.1974.sp010490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore D. I., Nastuk W. L. Effects of 'calcium ionophore' X537A on frog skeletal muscle. Nature. 1975 Feb 20;253(5493):644–646. doi: 10.1038/253644a0. [DOI] [PubMed] [Google Scholar]
- Hammerstad J. P., Murray J. E., Cutler R. W. Efflux of amino acid neurotransmitters from rat spinal cord slices. II. Factors influencing the electrically induced efflux of ( 14 C)glycine and 3 H-GABA. Brain Res. 1971 Dec 24;35(2):357–367. doi: 10.1016/0006-8993(71)90480-x. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Kelly J. S. Uptake and metabolism of gamma-aminobutyric acid by neurones and glial cells. Biochem Pharmacol. 1975 May 1;24(9):933–938. doi: 10.1016/0006-2952(75)90422-0. [DOI] [PubMed] [Google Scholar]
- Johnston G. A. Effects of calcium on the potassium-stimulated release of radioactive beta-alanine and gamma-aminobutyric acid from slices of rat cerebral cortex and spinal cord. Brain Res. 1977 Jan 31;121(1):179–181. doi: 10.1016/0006-8993(77)90449-8. [DOI] [PubMed] [Google Scholar]
- Katz R. I., Chase T. N., Kopin I. J. Effect of ions on stimulus-induced release of amino acids from mammalian brain slices. J Neurochem. 1969 Jun;16(3):961–967. doi: 10.1111/j.1471-4159.1969.tb08986.x. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G., Orkand R. K. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):768–787. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G. The physiology of neuroglial cells. Ergeb Physiol. 1966;57:1–90. [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy W. B., Haycock J. W., Cotman C. W. Effects of polyvalent cations on stimulus-coupled secretion of (14C)-gamma-aminobutyric acid from isolated brain synaptosomes. Mol Pharmacol. 1974 May;10(3):438–449. [PubMed] [Google Scholar]
- Mayevsky A., Zeuthen T., Chance B. Measurements of extracellular potassium, ECoG and pyridine nucleotide levels during cortical spreading depression in rats. Brain Res. 1974 Aug 16;76(2):347–349. doi: 10.1016/0006-8993(74)90467-3. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchin M. C. Factors influencing the efflux of [3H]gamma-aminobutyric acid from satellite glial cells in rat sensory ganglia. J Neurochem. 1975 Mar;24(3):571–577. doi: 10.1111/j.1471-4159.1975.tb07676.x. [DOI] [PubMed] [Google Scholar]
- Minchin M. C., Iversen L. L. Release of (3H)gamma-aminobutyric acid from glial cells in rat dorsal root ganglia. J Neurochem. 1974 Sep;23(3):533–540. doi: 10.1111/j.1471-4159.1974.tb06056.x. [DOI] [PubMed] [Google Scholar]
- Muller R. U., Finkelstein A. The effect of surface charge on the voltage-dependent conductance induced in thin lipid membranes by monazomycin. J Gen Physiol. 1972 Sep;60(3):285–306. doi: 10.1085/jgp.60.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkand R. K., Nicholls J. G., Kuffler S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Orrego F., Jankelevich J., Ceruti L., Ferrera E. Differential effects of electrical stimulation on release of 3H-noradrenaline and 14C-alpha-aminoisobutyrate from brain slices. Nature. 1974 Sep 6;251(5470):55–56. doi: 10.1038/251055a0. [DOI] [PubMed] [Google Scholar]
- PERRY W. L. M. Acetylcholine release in the cat's superior cervical ganglion. J Physiol. 1953 Mar;119(4):439–454. doi: 10.1113/jphysiol.1953.sp004858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape L. G., Katzman R. Response of glia in cat sensorimotor cortex to increased extracellular potassium. Brain Res. 1972 Mar 10;38(1):71–92. doi: 10.1016/0006-8993(72)90590-2. [DOI] [PubMed] [Google Scholar]
- Perri V., Sacchi O., Caella C. Electrical properties and synaptic connections of the sympathetic neurons in the rat and guinea-pig superior cervical ganglion. Pflugers Arch. 1970;314(1):40–54. doi: 10.1007/BF00587045. [DOI] [PubMed] [Google Scholar]
- Pfeiffer D. R., Reed P. W., Lardy H. A. Ultraviolet and fluorescent spectral properties of the divalent cation ionophore A23187 and its metal ion complexes. Biochemistry. 1974 Sep 10;13(19):4007–4014. doi: 10.1021/bi00716a029. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Properties of ionophores with broad range cation selectivity. Fed Proc. 1973 Jun;32(6):1698–1703. [PubMed] [Google Scholar]
- Prince D. A., Lux H. D., Neher E. Measurement of extracellular potassium activity in cat cortex. Brain Res. 1973 Feb 28;50(2):489–495. doi: 10.1016/0006-8993(73)90758-0. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Goldring S. Ionic determinants of membrane potential of cells presumed to be glia in cerebral cortex of cat. J Neurophysiol. 1973 Sep;36(5):855–868. doi: 10.1152/jn.1973.36.5.855. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Goldring S. Slow depolarization in cells presumed to be glia in cerebral cortex of cat. J Neurophysiol. 1973 Sep;36(5):869–878. doi: 10.1152/jn.1973.36.5.869. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Goldring S. Slow hyperpolarization in cells presumed to be glia in cerebral cortex of cat. J Neurophysiol. 1973 Sep;36(5):879–892. doi: 10.1152/jn.1973.36.5.879. [DOI] [PubMed] [Google Scholar]
- Redburn D. A., Shelton D., Cotman C. W. Calcium-dependent release of exogenously loaded gamma-amino-[U-14C]butyrate from synaptosomes: time course of stimulation by potassium, veratridine, and the calcium ionophore, A23187. J Neurochem. 1976 Feb;26(2):297–303. doi: 10.1111/j.1471-4159.1976.tb04480.x. [DOI] [PubMed] [Google Scholar]
- Roberts P. J. Amino acid release from isolated rat dorsal root ganglia. Brain Res. 1974 Jul 12;74(2):327–332. doi: 10.1016/0006-8993(74)90586-1. [DOI] [PubMed] [Google Scholar]
- Schon F., Kelly J. S. Selective uptake of (3H)beta-alanine by glia: association with glial uptake system for GABA. Brain Res. 1975 Mar 21;86(2):243–257. doi: 10.1016/0006-8993(75)90700-3. [DOI] [PubMed] [Google Scholar]
- Somjen G. G. Electrophysiology of neuroglia. Annu Rev Physiol. 1975;37:163–190. doi: 10.1146/annurev.ph.37.030175.001115. [DOI] [PubMed] [Google Scholar]
- Srinivasan V., Neal M. J., Mitchell J. F. The effect of electrical stimulation and high potassium concentrations on the efflux of (3H) gamma-aminobutyric acid from brain slices. J Neurochem. 1969 Aug;16(8):1235–1244. doi: 10.1111/j.1471-4159.1969.tb05971.x. [DOI] [PubMed] [Google Scholar]
- Walsh J. M., Bowery N. G., Brown D. A., Clark J. B. Metabolism of gamma-aminobutyric acid (GABA) by peripheral nervous tissue. J Neurochem. 1974 Jun;22(6):1145–1147. doi: 10.1111/j.1471-4159.1974.tb04350.x. [DOI] [PubMed] [Google Scholar]
- Young J. A., Brown D. A., Kelly J. S., Schon F. Autoradiographic localization of sites of (3H)gamma-aminobutyric acid accumulation in peripheral autonomic ganglia. Brain Res. 1973 Dec 7;63:479–486. doi: 10.1016/0006-8993(73)90128-5. [DOI] [PubMed] [Google Scholar]


