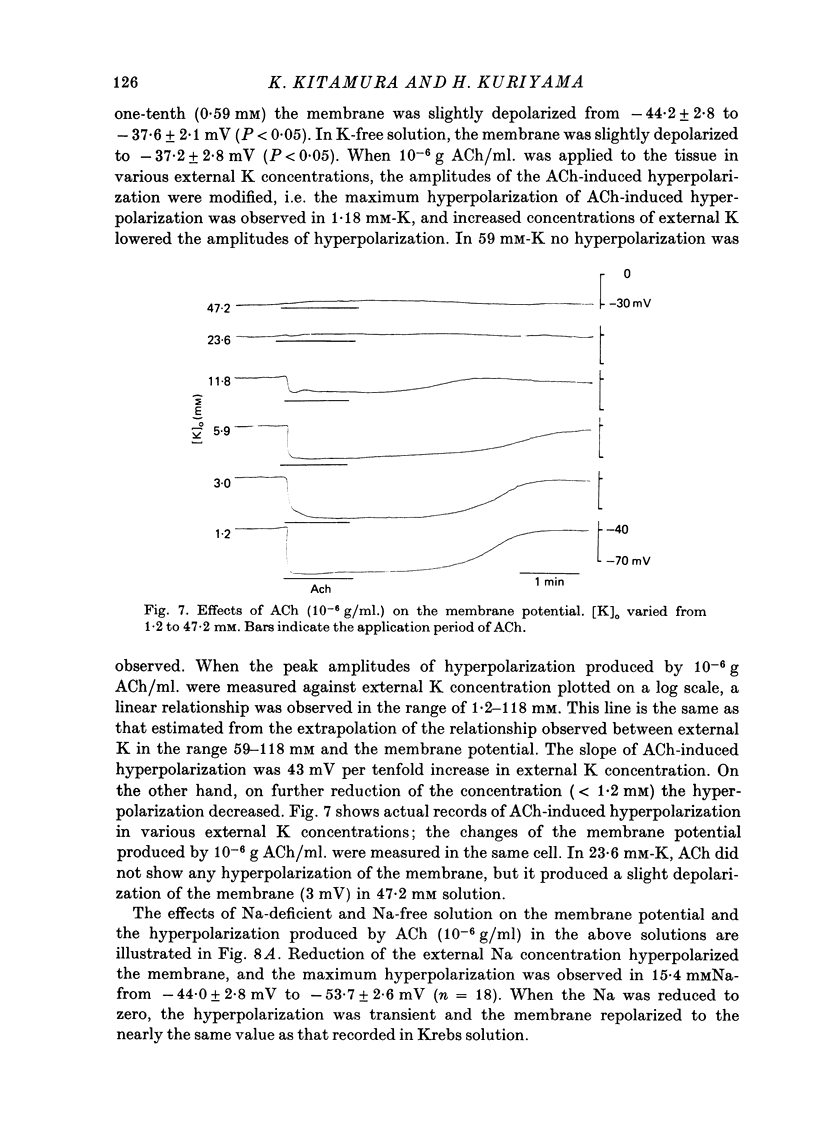
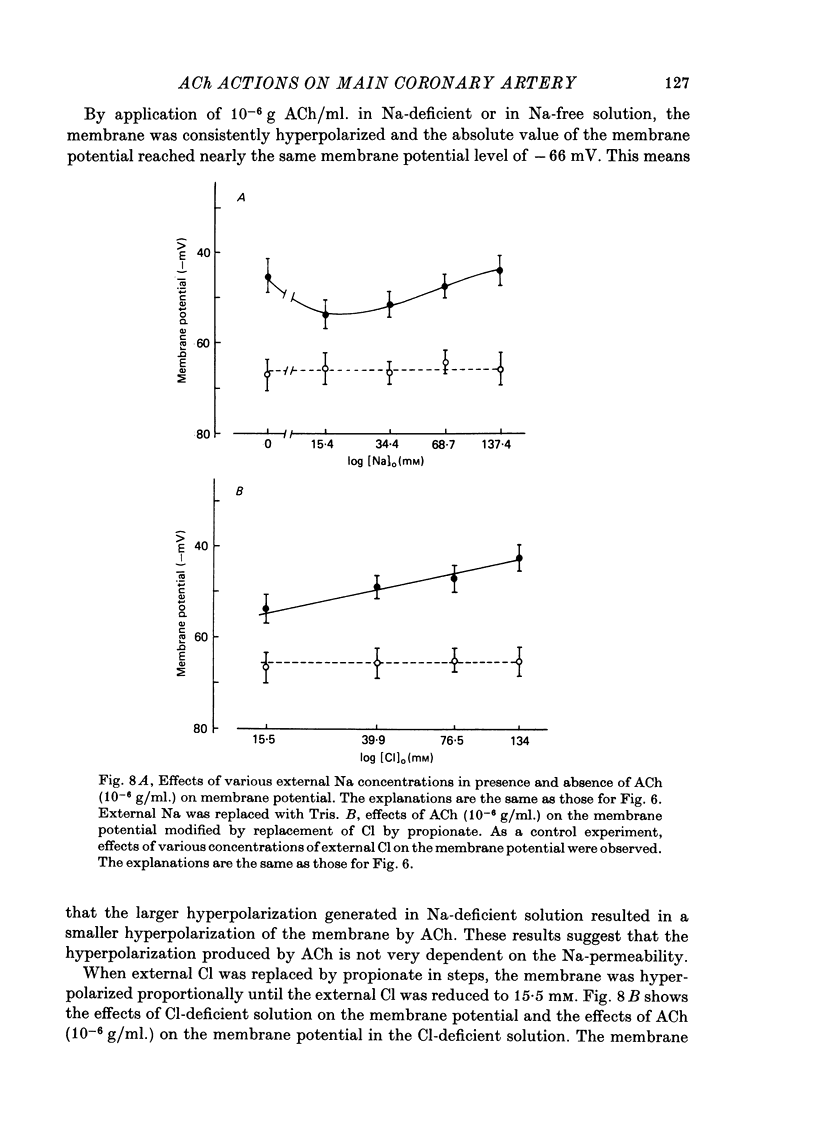
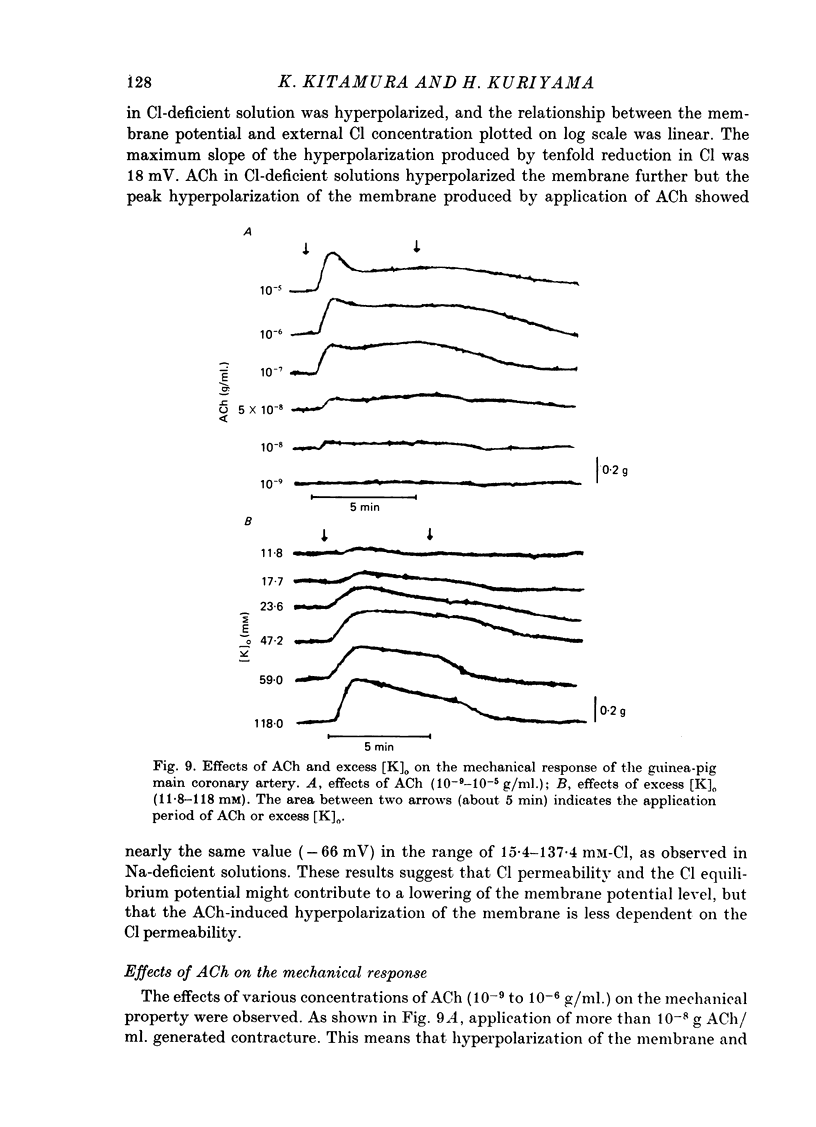
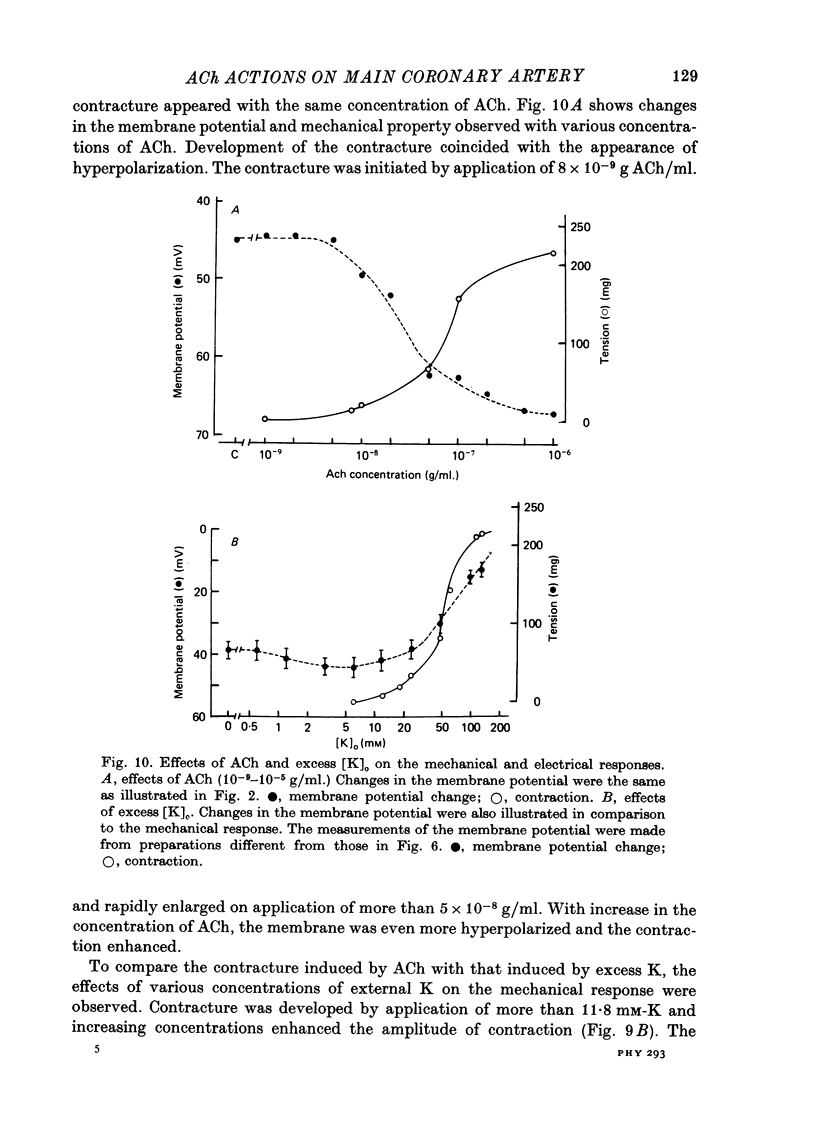
Abstract
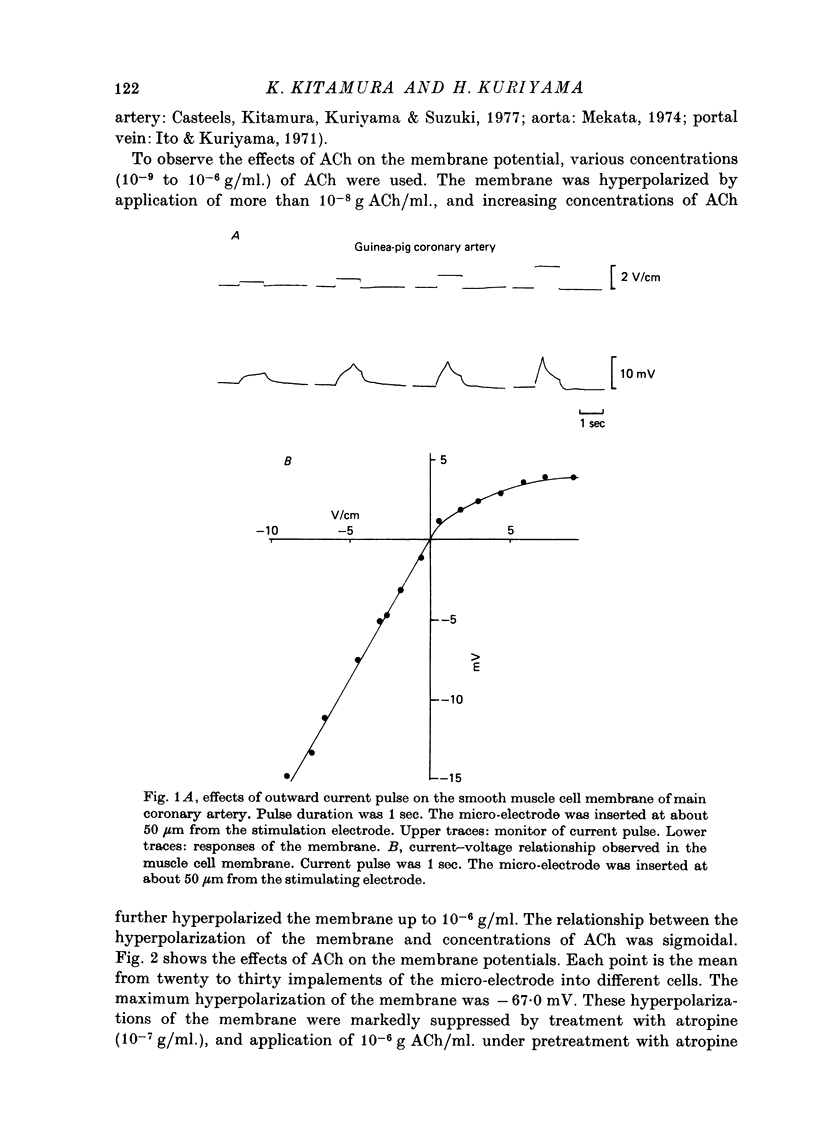
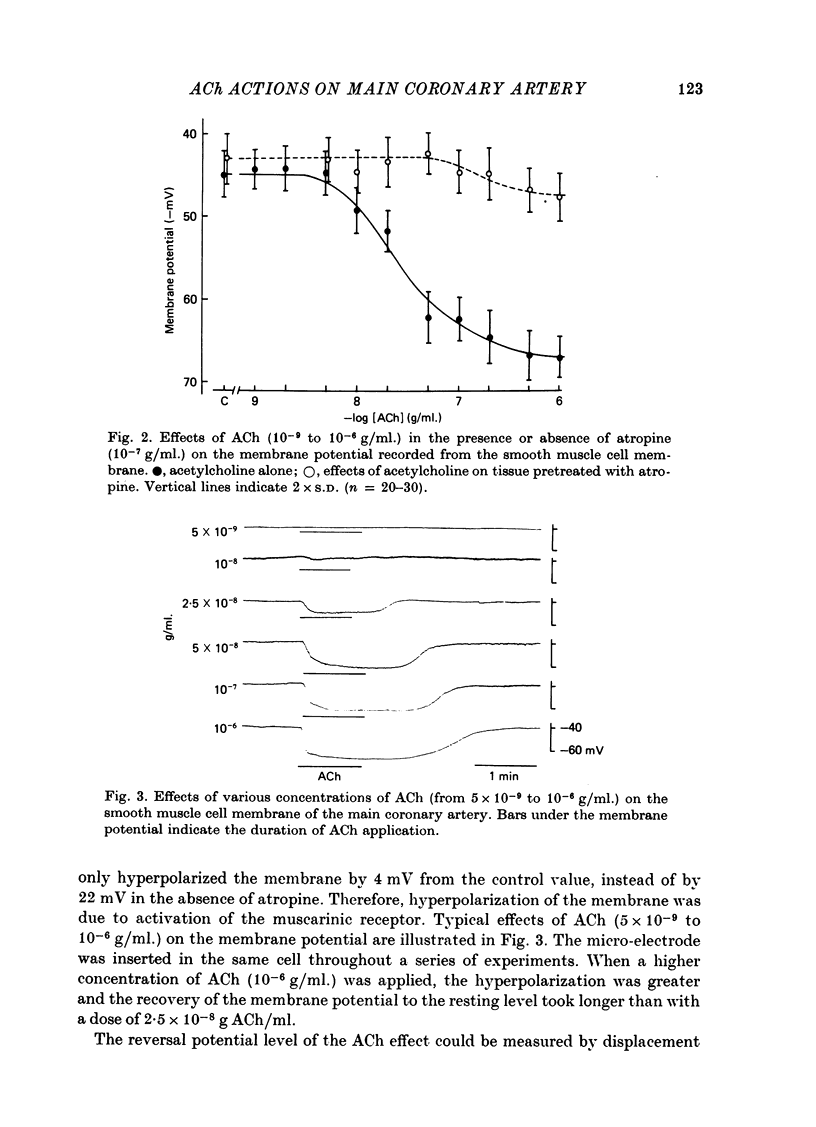
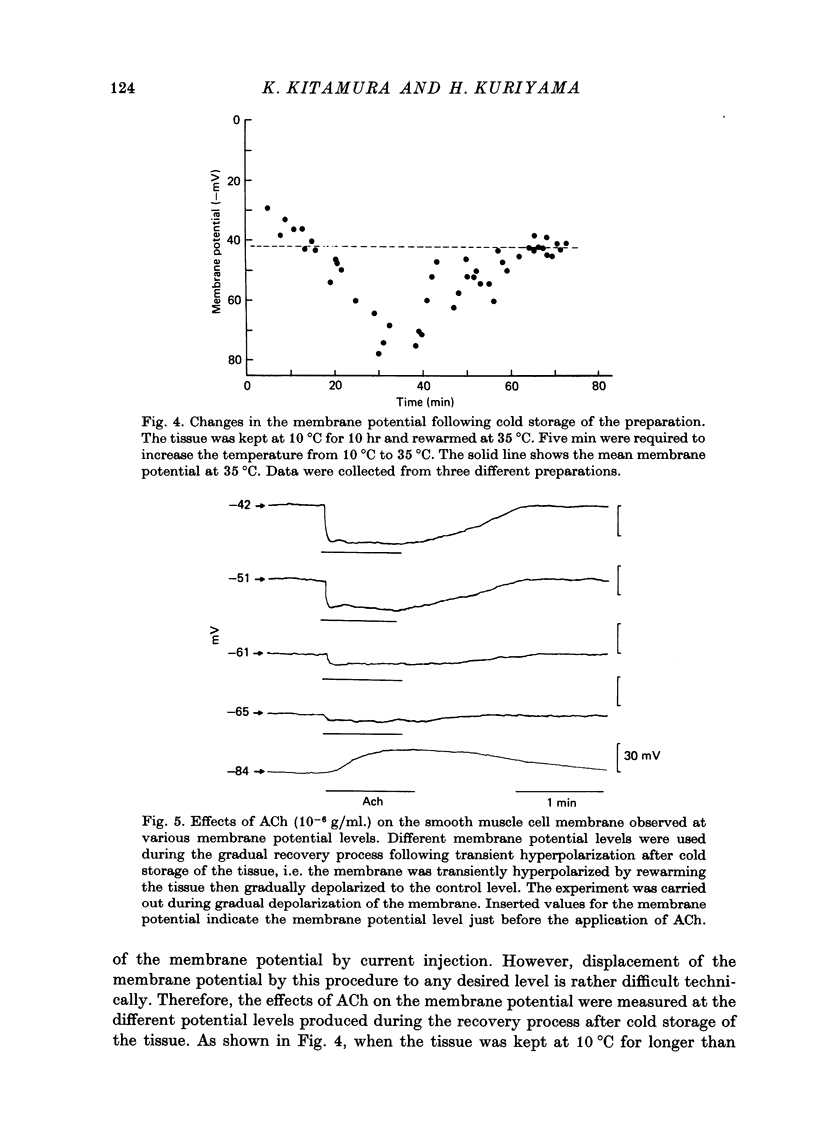
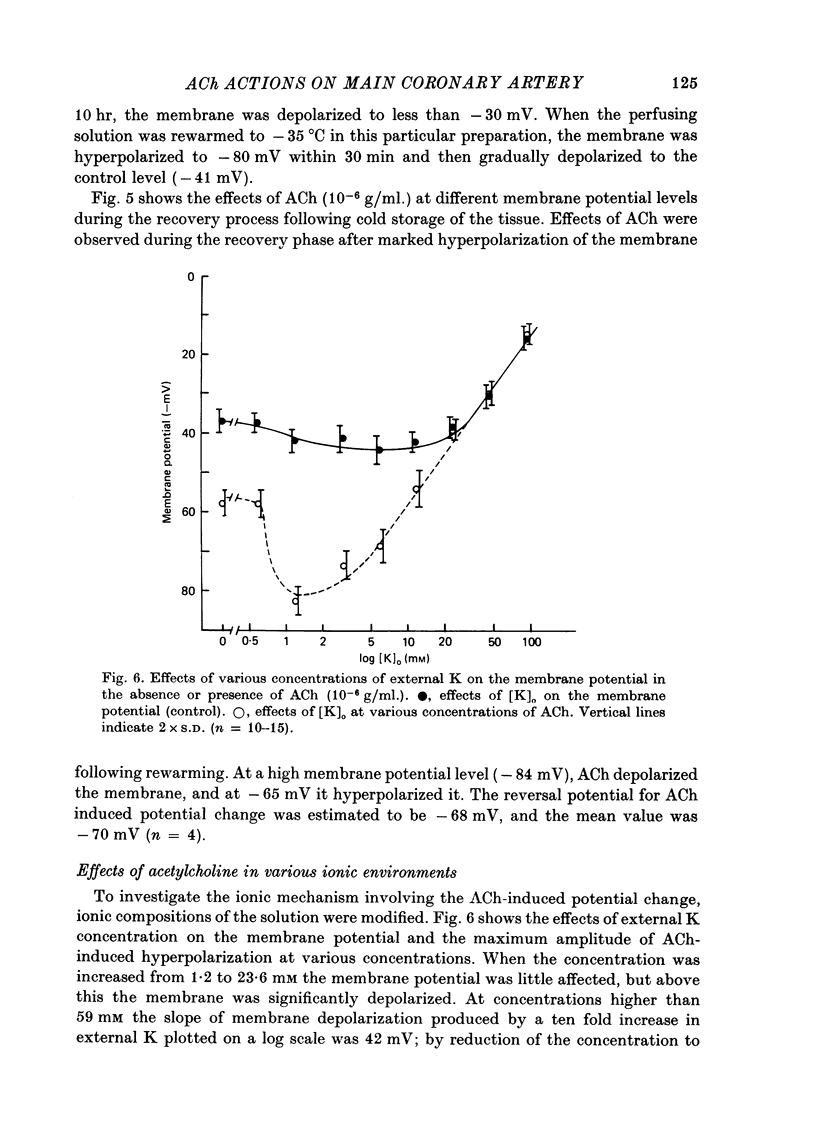
The effects of ACh on the smooth muscle cell membrane and mechanical property of the guinea-pig main coronary artery were observed by micro-electrode and isometric tension recording methods. 1. The membrane potential was low (--44 mV) and the membrane was electrically quiescent. Application of outward current pulse generated only a small graded response. The current--voltage relationship was linear for application of inward current pulses. 2. In low external Na or Cl solution the membrane was hyperpolarized. In external K solution of the 1.2--17.8 mM the membrane potential remained nearly the same; however increasing it to more than 29.5 mM depolarized the membrane. The maximum slope of depolarization was 42 mV per tenfold increase in external K. These results indicate that both Na and Cl equilibrium potentials were positive to the resting membrane potential. 3. ACh (greater than 10(-8) g/ml.) hyperpolarized the membrane, to a maximum of --67 mV with 10(-6) g ACh/ml. The dose--response relationship was sigmoidal, and the hyperpolarization was suppressed by atropine (10(-7) g/ml.). In external K of 1.2--29.5 mM, the application of 10(-6) g/ml. ACh hyperpolarized the membrane; this ACh-induced hyperpolarization was maximal in 1.2 mM-K (from --42.4 to --82.4 mV). When the hyperpolarization produced by 10(-6) g ACh/ml. was plotted against the external K concentration on a log scale, the relationship was linear above 1.2 mM-K and similar to that observed between the membrane potential and external K concentration between 29.5 and 118 mM in the absence of ACh. However, in a solution containing less than 1.2 mM-K, the amplitudes of ACh-induced hyperpolarization were reduced. 4. In Na- or Cl-deficient solution the membrane was hyperpolarized. The peak hyperpolarization to ACh was not modified by these changes in the ionic environments. It is concluded that ACh increases K permeability selectively in this muscle membrane. 5. To observe the reversal potential level of ACh-induced hyperpolarization, the effects of ACh were observed during the hyperpolarization and subsequent recovery of the membrane on rewarming the tissue following cold storage. When the membrane potential was high (less than --70 mV) ACh produced depolarization, but when it was low (greater than --70 mV) ACh produced hyperpolarization. The reversal potential level for ACh-induced potential change was about --70 mV. 6. Application of ACh (greater than 10(-8) g/ml.) evoked a mechanical response. The hyperpolarization of the membrane produced by ACh appeared coincidently with tension development. ACh also enhanced the amplitude of contracture produced by excess external K concentration. 7. It is concluded that ACh might increase K and Ca permeabilities of the membrane and release Ca from the intracellular store, thus causing hyperpolarization of the membrane and contraction.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Membrane potentials of smooth muscle fibres of the taenia coli of the guinea-pig. J Physiol. 1954 Aug 27;125(2):302–315. doi: 10.1113/jphysiol.1954.sp005159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURN J. H., RAND M. J. ACETYLCHOLINE IN ADRENERGIC TRANSMISSION. Annu Rev Pharmacol. 1965;5:163–182. doi: 10.1146/annurev.pa.05.040165.001115. [DOI] [PubMed] [Google Scholar]
- Bevan J. A., Su C. Sympathetic mechanisms in blood vessels: nerve and muscle relationships. Annu Rev Pharmacol. 1973;13:269–285. doi: 10.1146/annurev.pa.13.040173.001413. [DOI] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. The membrane properties of the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):41–61. doi: 10.1113/jphysiol.1977.sp011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- FUNAKI S., BOHR D. F. ELECTRICAL AND MECHANICAL ACTIVITY OF ISOLATED VASCULAR SMOOTH MUSCLE OF THE RAT. Nature. 1964 Jul 11;203:192–194. doi: 10.1038/203192b0. [DOI] [PubMed] [Google Scholar]
- Feigl E. O. Parasympathetic control of coronary blood flow in dogs. Circ Res. 1969 Nov;25(5):509–519. doi: 10.1161/01.res.25.5.509. [DOI] [PubMed] [Google Scholar]
- Holman M. E. Electrophysiology of vascular smooth muscle. Ergeb Physiol. 1969;61:137–177. doi: 10.1007/BFb0111448. [DOI] [PubMed] [Google Scholar]
- Hume W. R., De la Lande I. S., Waterson J. G. Effect of acetylcholine on the response of the isolated rabbit ear artery to stimulation of the perivascular sympathetic nerves. Eur J Pharmacol. 1972 Feb;17(2):227–233. doi: 10.1016/0014-2999(72)90163-x. [DOI] [PubMed] [Google Scholar]
- Ito Y., Kuriyama H. Membrane properties of the smooth-muscle fibres of the guinea-pig portal vein. J Physiol. 1971 May;214(3):427–441. doi: 10.1113/jphysiol.1971.sp009442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Suzuki H., Kuriyama H. On the roles of calcium ion during potassium induced contracture in the smooth muscle cells of the rabbit main pulmonary artery. Jpn J Physiol. 1977;27(6):755–770. doi: 10.2170/jjphysiol.27.755. [DOI] [PubMed] [Google Scholar]
- Keatinge W. R. Electrical and mechanical response of arteries to stimulation of sympathetic nerves. J Physiol. 1966 Aug;185(3):701–715. doi: 10.1113/jphysiol.1966.sp008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. Electrical property and chemical sensitivity of vascular smooth muscles in normotensive and spontaneously hypersensitive rats. J Physiol. 1978 Dec;285:409–424. doi: 10.1113/jphysiol.1978.sp012579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik K. U., Ling G. M. Modification by acetylcholine of the response of rat mesenteric arteries to sympathetic stimulation. Circ Res. 1969 Jul;25(1):1–9. doi: 10.1161/01.res.25.1.1. [DOI] [PubMed] [Google Scholar]
- Mekata F. Current spread in the smooth muscle of the rabbit aorta. J Physiol. 1974 Oct;242(1):143–155. doi: 10.1113/jphysiol.1974.sp010698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata H., Niu H. Electrical and mechanical responses of coronary artery smooth muscle to catecholamines. Jpn J Physiol. 1969 Oct 15;19(5):599–608. doi: 10.2170/jjphysiol.19.599. [DOI] [PubMed] [Google Scholar]
- Nakajima A., Horn L. Electrical activity of single vascular smooth muscle fibers. Am J Physiol. 1967 Jul;213(1):25–30. doi: 10.1152/ajplegacy.1967.213.1.25. [DOI] [PubMed] [Google Scholar]
- Norton J. M., Detar R. Potassium and isolated coronary vascular smooth muscle. Am J Physiol. 1972 Feb;222(2):474–479. doi: 10.1152/ajplegacy.1972.222.2.474. [DOI] [PubMed] [Google Scholar]
- Rice A. J., Long J. P. An unusual venoconstriction induced by acetylcholine. J Pharmacol Exp Ther. 1966 Mar;151(3):423–429. [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther. 1968 Jan;159(1):129–145. [PubMed] [Google Scholar]
- Steinsland O. S., Furchgott R. F., Kirpekar S. M. Inhibition of adrenergic neurotransmission by parasympathomimetics in the rabbit ear artery. J Pharmacol Exp Ther. 1973 Feb;184(2):346–356. [PubMed] [Google Scholar]
- Su C., Bevan J. A. The electrical response of pulmonary artery muscle to acetylcholine, histamine and serotonin. Life Sci. 1965 May;4(10):1025–1029. doi: 10.1016/0024-3205(65)90221-3. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Lorenz R. R., Tyce G. M. Inhibition of norepinephrine- 3 H release from sympathetic nerve endings in veins by acetylcholine. J Pharmacol Exp Ther. 1973 May;185(2):386–394. [PubMed] [Google Scholar]



