Abstract
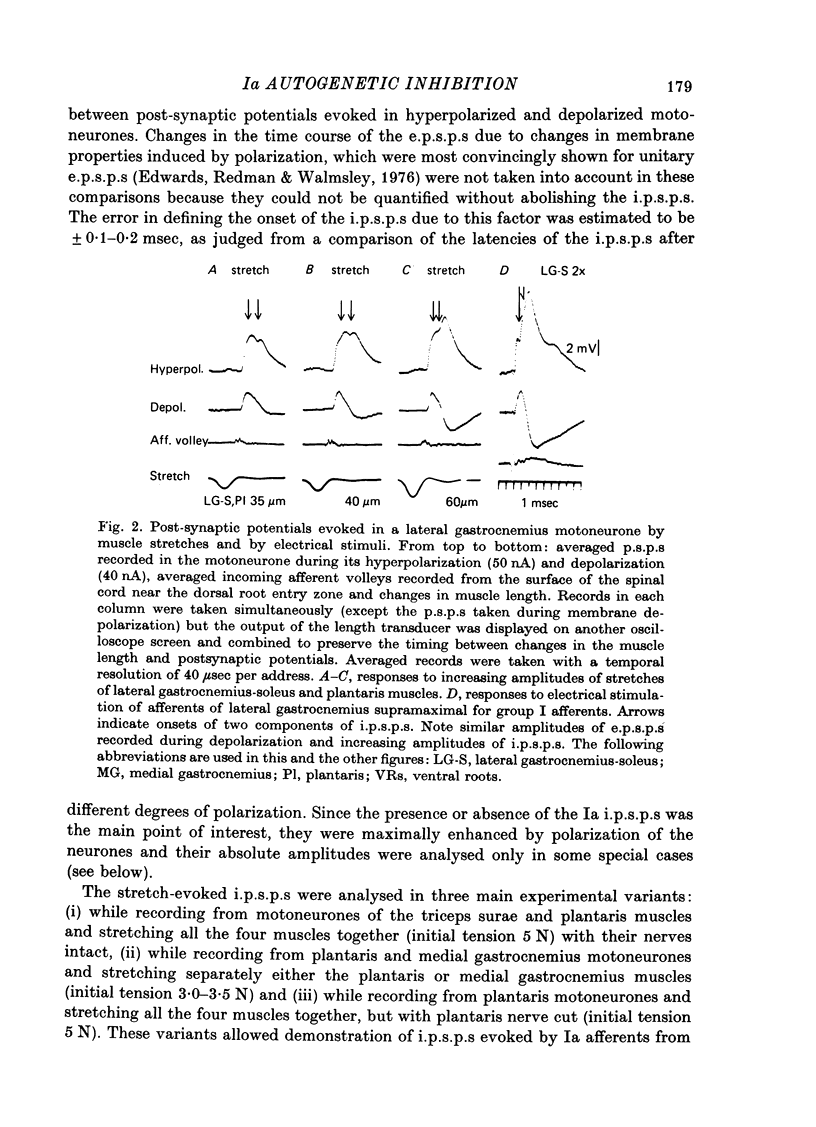
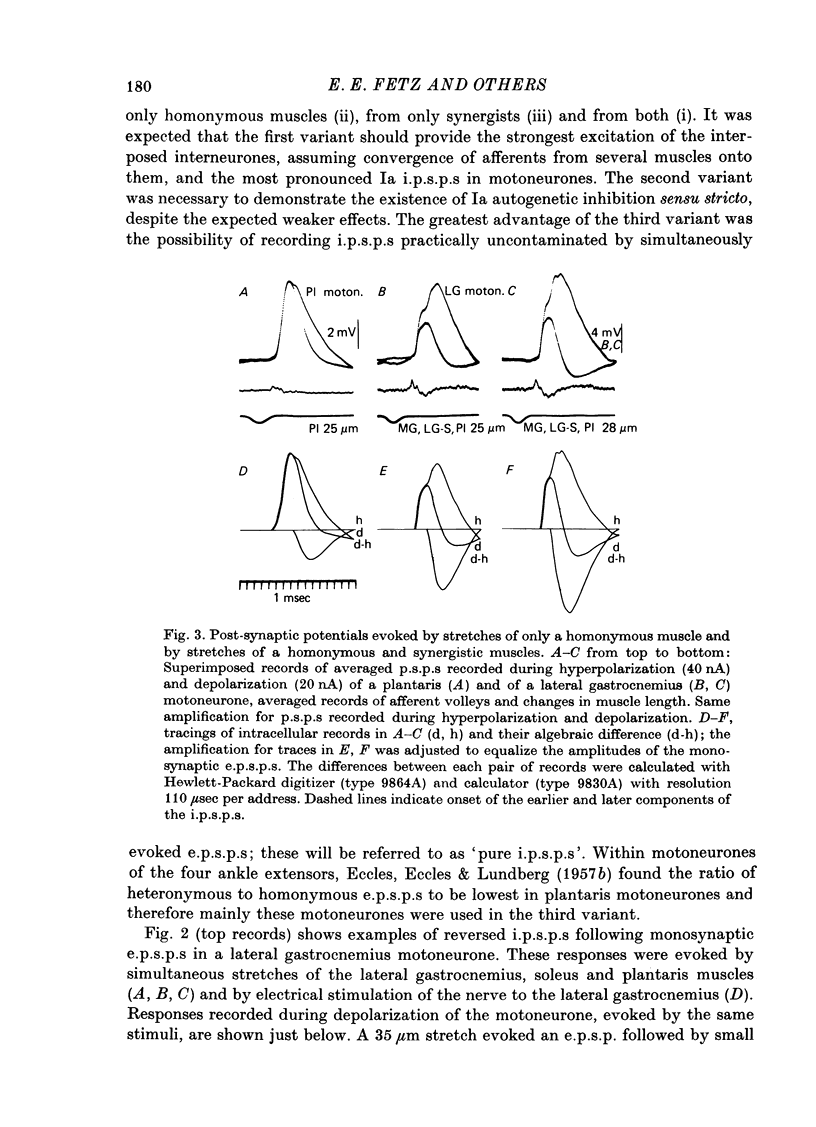
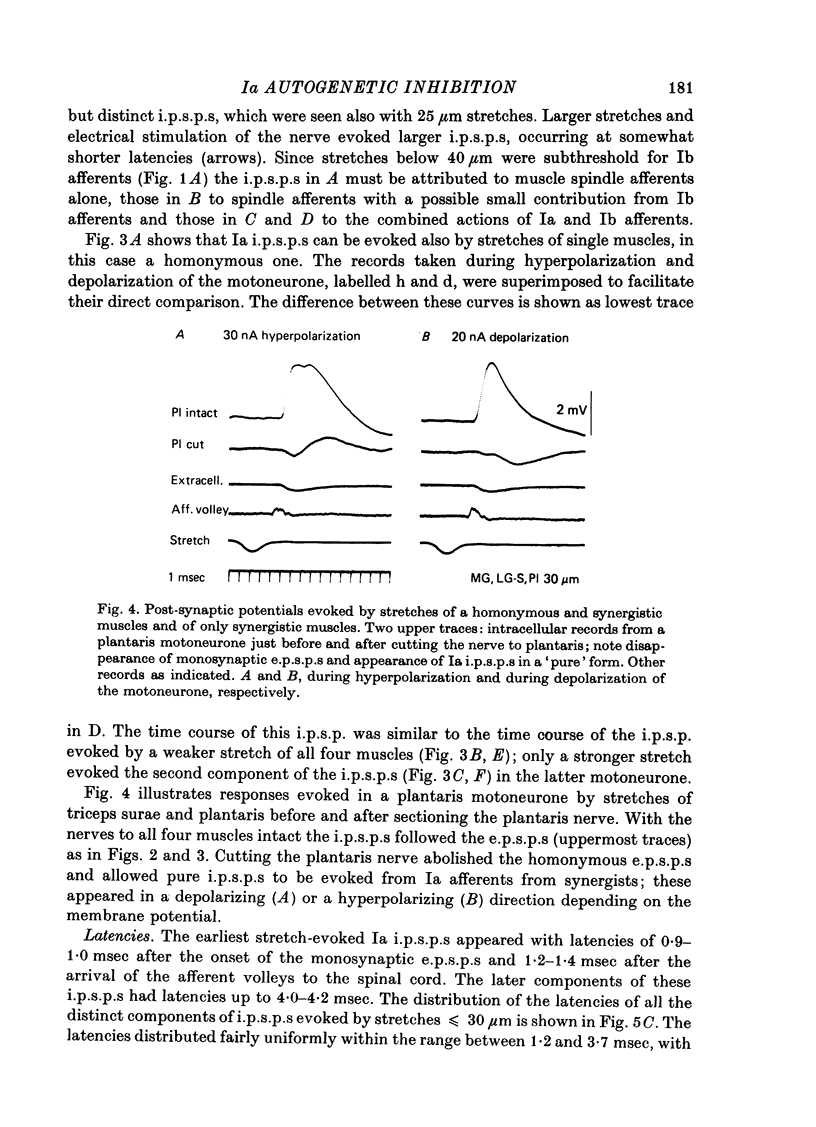
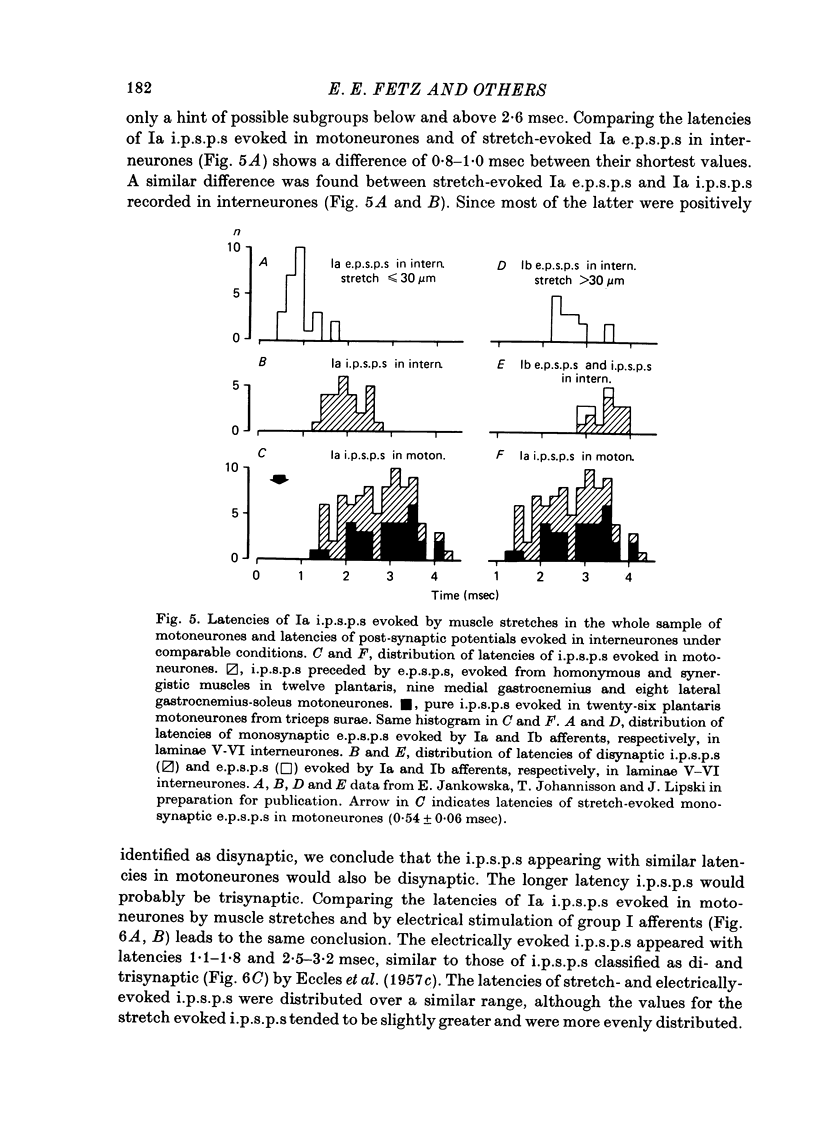
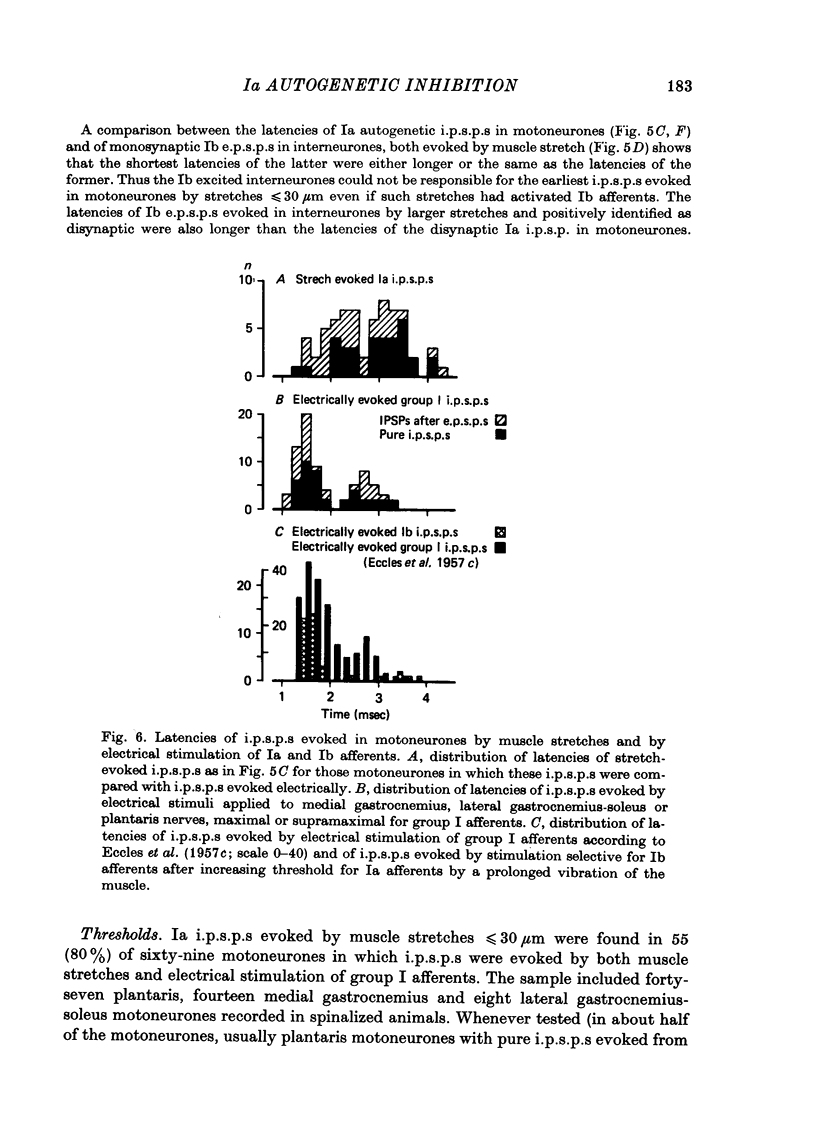
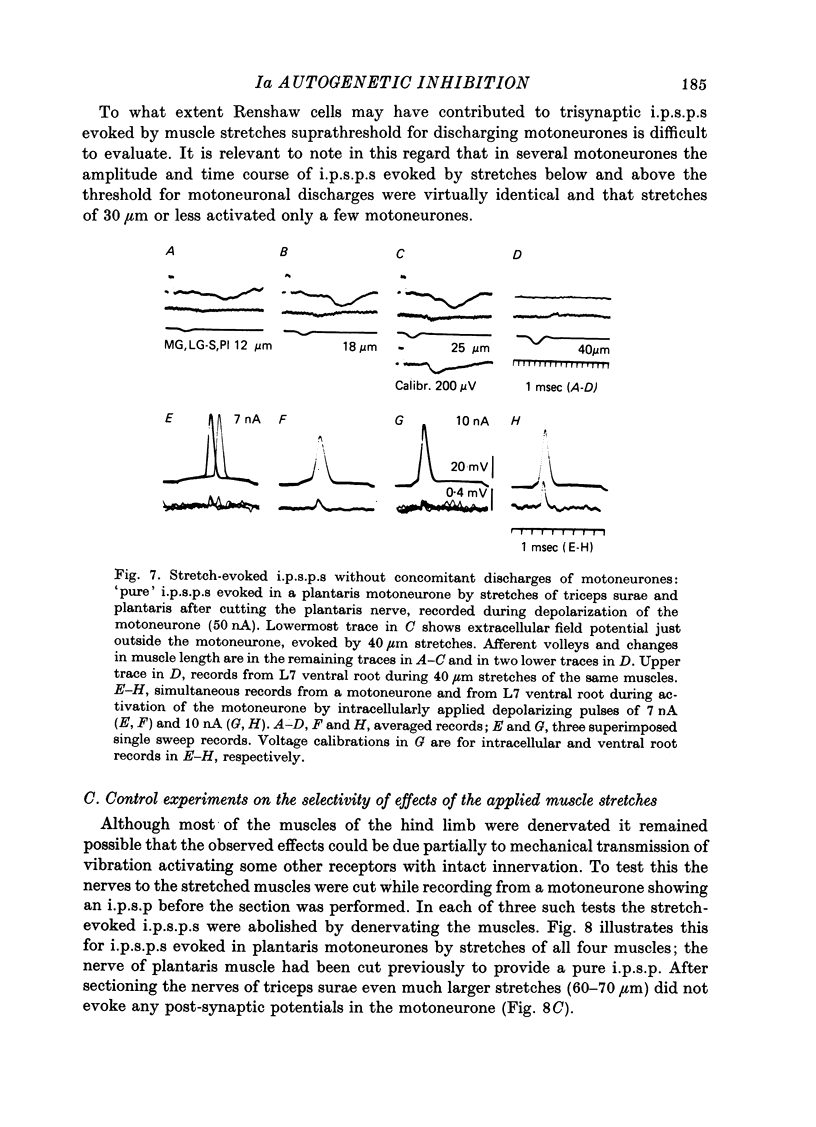
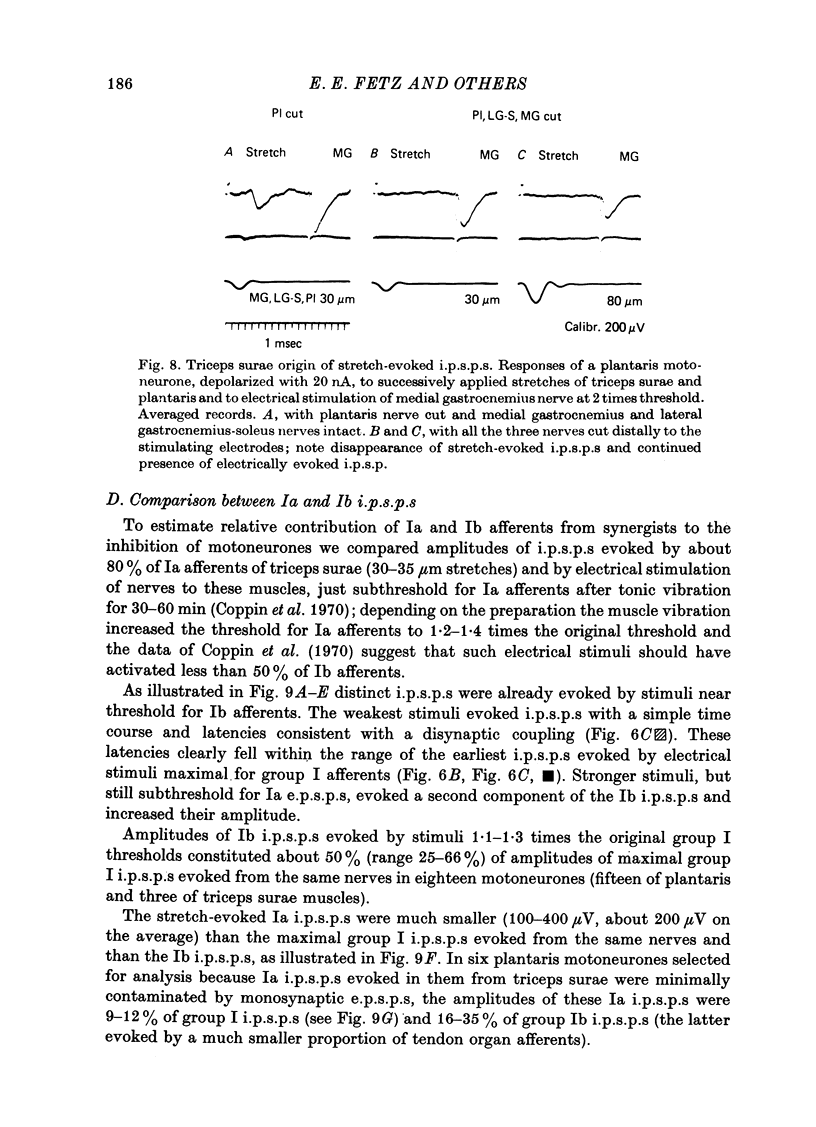
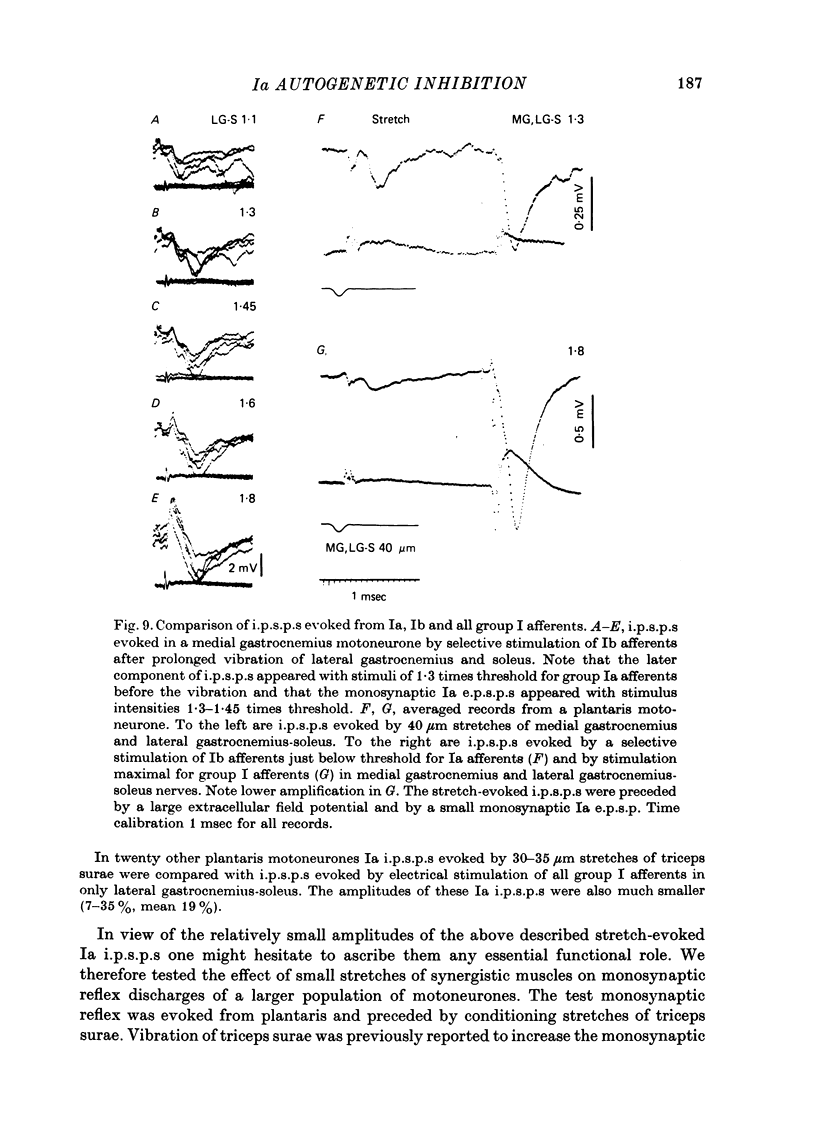
1. Inhibitory post-synaptic potentials evoked by adequate stimulation of group Ia muscle spindle afferents of homonymous and synergistic muscles and by selective electrical stimulation of tendon organ afferents were analysed in motoneurones of triceps surae and plantaris. 2. Selective activation of Ia afferents was verified to occur with brief stretches of triceps surae and plantaris 35 micrometer or less in amplitude with an initial muscle tension of 5 N; stretches of 30--35 micrometer were estimated to activate 80--90% of Ia afferents in these muscles. Under the same conditions the lowest thresholds for group Ib tendon organ afferents were about 40 micrometer. 3. Stretches less than or equal to 30 micrometer evoked i.p.s.p.s in 80% of triceps surae and plantaris motoneurones; lowest thresholds for evoking i.p.s.p.s wef triceps surae and plantaris motoneurones; lowest thresholds for evoking i.p.s.p.s were 10 micrometer or less. However, such low thresholds for stretch-evoked i.p.s.p.s, lower than the thresholds for activation of Ib afferents, were found mainly in spinalized, unanaesthetized (after decerebration) or lightly anaesthetized animals. The latencies of these i.p.s.p.s indicated disynaptic and trisynaptic coupling between Ia afferents and motoneurones. The i.p.s.p.s were evoked (i) from the homonymous and synergistic muscles stretched together, (ii) from the homonymous muscles alone and (iii) from the synergistic muscles alone. 4. Control experiments showed that i.p.s.p.s could be evoked by stretches sub-threshold for discharging motoneurones, thus showing that those i.p.s.p.s were not mediated by Renshaw cells. The stretch-evoked i.p.s.p.s disappeared after sectioning the nerves from the corresponding muscles, further excluding their mediation by afferents other than group Ia afferents from thf stretched muscle. 5. In order to selectively activate tendon organ afferents, thresholds for excitation of Ia afferents by electrical stimuli were increased to a level above the threshold for Ib afferents by prolonged muscle vibration (Coppin, Jack & MacLennan, 1970). I.p.s.p.s evoked by stimuli near threshold for Ib afferents appeared with latencies indicating disynaptic coupling. Later (trisynaptic) components of Ib i.p.s.p.s required somewhat stronger stimuli. 6. Amplitudes of Ia i.p.s.p.s evoked by muscle stretches activating about 80% of muscle spindle afferents were compared with amplitudes of Ib i.p.s.p.s due to less than 50% of tendon organ afferents of the same muscles. The Ia i.p.s.p.s were much smaller (16--35%) than the Ib i.p.s.p.s. The amplitudes of such Ia and Ib i.p.s.p.s constituted about 10 and 25--66%, respectively, of the maximal i.p.s.p.s evoked by electrical stimulation of all group I afferents. 7. We conclude that inhibition of motoneurones may be evoked from Ia muscle spindle afferents from homonymous and synergistic muscles as well as from Ib tendon organ afferents...
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andén N. E., Jukes M. G., Lundberg A., Vyklický L. The effect of DOPA on the spinal cord. 1. Influence on transmission from primary afferents. Acta Physiol Scand. 1966 Jul-Aug;67(3):373–386. doi: 10.1111/j.1748-1716.1966.tb03324.x. [DOI] [PubMed] [Google Scholar]
- BIANCONI R., GRANIT R., REIS D. J. THE EFFECTS OF EXTENSOR MUSCLE SPINDLES AND TENDON ORGANS ON HOMONYMOUS MOTONEURONES IN RELATION TO GAMMA-BIAS AND CURARIZATION. Acta Physiol Scand. 1964 Aug;61:331–347. [PubMed] [Google Scholar]
- BROCK L. G., COOMBS J. S., ECCLES J. C. The recording of potentials from motoneurones with an intracellular electrode. J Physiol. 1952 Aug;117(4):431–460. doi: 10.1113/jphysiol.1952.sp004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Engberg I., Matthews P. B. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967 Oct;192(3):773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. Excitatory synaptic action in motoneurones. J Physiol. 1955 Nov 28;130(2):374–395. doi: 10.1113/jphysiol.1955.sp005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarkowska J., Jankowska E., Sybirska E. Axonal projections of spinal interneurones excited by group I afferents in the cat, revealed by intracellular staining with horseradish peroxidase. Brain Res. 1976 Dec 10;118(1):115–118. doi: 10.1016/0006-8993(76)90844-1. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J Physiol. 1957 Sep 30;138(2):227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones in relation to the two components of the group I muscle afferent volley. J Physiol. 1957 May 23;136(3):527–546. doi: 10.1113/jphysiol.1957.sp005778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Types of neurone in and around the intermediate nucleus of the lumbosacral cord. J Physiol. 1960 Nov;154:89–114. doi: 10.1113/jphysiol.1960.sp006566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958 Dec 4;144(2):271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Supraspinal control of interneurones mediating spinal reflexes. J Physiol. 1959 Oct;147:565–584. doi: 10.1113/jphysiol.1959.sp006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. The effect of polarizing currents on unitary Ia excitatory post-synaptic potentials evoked in spinal motoneurones. J Physiol. 1976 Aug;259(3):705–723. doi: 10.1113/jphysiol.1976.sp011490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Trott J. R. Autogenetic reflex action on to gamma motoneurones by stretch of triceps surae in the decerebrated cat. J Physiol. 1978 Mar;276:49–66. doi: 10.1113/jphysiol.1978.sp012219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Trott J. R. Reflex connections form muscle stretch receptors to their own fusimotor neurones. Prog Brain Res. 1976;44:113–122. doi: 10.1016/S0079-6123(08)60727-X. [DOI] [PubMed] [Google Scholar]
- Emonet-Dénand F., Laporte Y. Proportion of muscles spindles supplied by skeletofusimotor axons (beta-axons) in peroneus brevis muscle of the cat. J Neurophysiol. 1975 Nov;38(6):1390–1394. doi: 10.1152/jn.1975.38.6.1390. [DOI] [PubMed] [Google Scholar]
- Engberg I., Lundberg A., Ryall R. W. Reticulospinal inhibition of transmission in reflex pathways. J Physiol. 1968 Jan;194(1):201–223. doi: 10.1113/jphysiol.1968.sp008402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm C., Noth J. Reflex responses of gamma motoneurones to vibration of the muscle they innervate. J Physiol. 1976 Mar;256(1):117–136. doi: 10.1113/jphysiol.1976.sp011315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., HENATSCH H. D. Gamma control of dynamic properties of muscle spindles. J Neurophysiol. 1956 Jul;19(4):356–366. doi: 10.1152/jn.1956.19.4.356. [DOI] [PubMed] [Google Scholar]
- GRANIT R. Reflex self-regulation of muscle contraction and autogenetic inhibition. J Neurophysiol. 1950 Sep;13(5):351–372. doi: 10.1152/jn.1950.13.5.351. [DOI] [PubMed] [Google Scholar]
- Grillner S., Hongo T., Lund S. Descending monosynaptic and reflex control of gamma-motoneurones. Acta Physiol Scand. 1969 Apr;75(4):592–613. doi: 10.1111/j.1748-1716.1969.tb04414.x. [DOI] [PubMed] [Google Scholar]
- HUNT C. C. The effect of stretch receptors from muscle on the discharge of motorneurons. J Physiol. 1952 Jul;117(3):359–379. doi: 10.1113/jphysiol.1952.sp004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase J., Cleveland S., Ross H. G. Problems of postsynaptic autogenous and recurrent inhibition in the mammalian spinal cord. Rev Physiol Biochem Pharmacol. 1975;73:73–129. doi: 10.1007/BFb0034660. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Convergence of excitatory and inhibitory action on interneurones in the lumbosacral cord. Exp Brain Res. 1966;1(4):338–358. doi: 10.1007/BF00237706. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition of interneurones monosynaptically activated from group Ia afferents. J Physiol. 1971 Jul;215(3):613–636. doi: 10.1113/jphysiol.1971.sp009488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Padel Y., Tanaka R. The mode of activation of pyramidal tract cells by intracortical stimuli. J Physiol. 1975 Aug;249(3):617–636. doi: 10.1113/jphysiol.1975.sp011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Monosynaptic excitation of motoneurones from muscle spindle secondary endings of intercostal and triceps surae muscles in the cat. J Physiol. 1975 Feb;245(2):64P–66P. [PubMed] [Google Scholar]
- Kuno M., Llinás R. Alterations of synaptic action in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):823–838. doi: 10.1113/jphysiol.1970.sp009244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAPORTE Y., LLOYD D. P. C. Nature and significance of the reflex connections established by large afferent fibers of muscular origin. Am J Physiol. 1952 Jun;169(3):609–621. doi: 10.1152/ajplegacy.1952.169.3.609. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., WINSBURY G. Selective adequate activation of large afferents from muscle spindles and Golgi tendon organs. Acta Physiol Scand. 1960 Jul 15;49:155–164. doi: 10.1111/j.1748-1716.1960.tb01939.x. [DOI] [PubMed] [Google Scholar]
- Laporte Y., Emonet-Dénand F. The skeleto-fusimotor innervation of cat muscle spindle. Prog Brain Res. 1976;44:99–109. doi: 10.1016/s0079-6123(08)60726-8. [DOI] [PubMed] [Google Scholar]
- Lucas M. E., Willis W. D. Identification of muscle afferents which activate interneurons in the intermediate nucleus. J Neurophysiol. 1974 Mar;37(2):282–293. doi: 10.1152/jn.1974.37.2.282. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Cutaneous facilitation of transmission in reflex pathways from Ib afferents to motoneurones. J Physiol. 1977 Mar;265(3):763–780. doi: 10.1113/jphysiol.1977.sp011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magherini P. C., Pompeiano O., Thoden U. The relative significance of presynaptic and postsynaptic effects on monosynaptic extensor reflexes during vibration of synergic muscles. Arch Ital Biol. 1972 May;110(1):70–89. [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Scott J. G., Mendell L. M. Individual EPSPs produced by single triceps surae Ia afferent fibers in homonymous and heteronymous motoneurons. J Neurophysiol. 1976 Jul;39(4):679–692. doi: 10.1152/jn.1976.39.4.679. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I., Kurchavyi G. G. Effects of trans-membrane polarization and TEA injection on monosynaptic actions from motor cortex, red nucleus and group Ia afferents on lumbar motoneurons in the monkey. Brain Res. 1974 Dec 20;82(1):49–67. doi: 10.1016/0006-8993(74)90892-0. [DOI] [PubMed] [Google Scholar]
- Smith T. G., Wuerker R. B., Frank K. Membrane impedance changes during synaptic transmission in cat spinal motoneurons. J Neurophysiol. 1967 Sep;30(5):1072–1096. doi: 10.1152/jn.1967.30.5.1072. [DOI] [PubMed] [Google Scholar]
- Stuart D. G., Mosher C. G., Gerlach R. L., Reinking R. M. Selective activation of Ia afferents by transient muscle stretch. Exp Brain Res. 1970 Jun 25;10(5):477–487. doi: 10.1007/BF00234264. [DOI] [PubMed] [Google Scholar]
- Stuart D. G., Willis W. D., Jr, Reinking R. M. Stretch-evoked excitatory postsynaptic potentials in motoneurons. Brain Res. 1971 Oct 8;33(1):115–125. doi: 10.1016/0006-8993(71)90310-6. [DOI] [PubMed] [Google Scholar]
- Taylor A., Watt D. G., Stauffer E. K., Reinking R. M., Stuart D. G. Use of afferent triggered averaging to study the central connections of muscle spindle afferents. Prog Brain Res. 1976;44:171–183. doi: 10.1016/S0079-6123(08)60732-3. [DOI] [PubMed] [Google Scholar]
- Werman R., Carlen P. L. Unusual behavior of the La EPSP in cat spinal motoneurons. Brain Res. 1976 Aug 13;112(2):395–401. doi: 10.1016/0006-8993(76)90294-8. [DOI] [PubMed] [Google Scholar]


