Abstract
Cryptococcus neoformans and cryptococcal surface polysaccharides influenced C5aR expression on human polymorphonuclear neutrophils (PMN). Encapsulated and acapsular strains produced dramatically different effects. Treatment of PMN with acapsular cryptococci up-regulated C5aR expression; whereas treatment with encapsulated cells suppressed C5aR expression. Glucuronoxylomannan (GXM), the principal constituent of the cryptococcal capsule, was responsible for such inhibition. Increased C5aR expression following treatment with acapsular cryptococci was accompanied by increased binding of C5a to PMN, increased superoxide production in response to stimulation with C5a, and an increased chemotactic response to C5a. Conversely, decreased C5aR expression following treatment with encapsulated cryptococci or acapsular cryptococci that had been pretreated with GXM was accompanied by decreased binding of C5a to PMN and a decreased chemotactic response to C5a. Our results raise the possibility that the down-regulation of C5aR expression by encapsulated cryptococci might alter PMN function at the site of cryptococcal infection.
The complement system is a major element of the humoral defense system. C3a, C4a, and C5a anaphylotoxins are active mediators generated by activation of the complement cascade (14). C5a is one of the best described and most potent proinflammatory mediators derived from the complement system (8); it induces a number of diverse functions in cellular systems, especially in polymorphonuclear neutrophils (PMN). These activities include stimulation of chemotaxis, aggregation, degranulation, production of superoxide anion, and increased mobilization of intracellular calcium (16, 23). The anaphylatoxins interact specifically with C5aR, a G-protein-coupled receptor expressed on human neutrophils, eosinophils, basophils, hepatocytes, and astrocytes (7, 33).
The encapsulated yeast Cryptococcus neoformans is a potent activator of the complement system. Incubation of encapsulated cryptococci in normal human serum (HS) leads to the deposition of 107 to 108 molecules of C3b/iC3b per yeast cell (29, 45). This activation occurs entirely via the action of the alternative complement pathway (30). Incubation of cryptococci in HS also leads to the generation of soluble cleavage fragments, most notably C5a, that are chemotactic for neutrophils (9, 31).
The importance of the complement system in host defense against fungal infection has been demonstrated by use of experimental animals with complement deficiencies. C5-deficient mice have reduced resistance to intravenous challenge and display altered pulmonary clearance of C. neoformans compared with C5-sufficient mice (32). C5-deficient mice also show decreased resistance to disseminated infection by Candida albicans, Aspergillus fumigatus, and Saccharomyces cerevisiae (1, 20). Resistance to experimental cryptococcosis (10, 17), candidiasis (15), and paracoccidioidomycosis (2) is reduced in animals treated with cobra venom factor. Cobra venom factor forms an extremely stable C3 convertase that leads to the rapid depletion of C3 and C5 levels in vivo (39). In a previous study, it was demonstrated that PMN from normal subjects released interleukin 8 (IL-8) in response to treatment with whole yeast cells of C. neoformans or the major constituent of the cryptococcal capsule, glucuronoxylomannan (GXM) (37). Further, this effect was found to be dependent on the generation of C3a and C5a (42). In contrast, PMN from patients with late-stage human immunodeficiency virus infection produced a significantly lower level of IL-8 after exposure to C. neoformans. This reduced level of IL-8 secretion correlated with a selective defect in C5aR (CD88) expression on PMN (33).
Many biological effects of neutrophils, including proinflammatory cytokine production (4, 33, 41), antimicrobial activity (36), and intracellular calcium mobilization (16, 23), are greatly influenced by the proinflammatory mediator C5a. Given the essential role that C5aR plays in the stimulation of neutrophils by C5a, we examined the effect of the treatment of neutrophils with C. neoformans and its major capsular polysaccharide, GXM, on the expression of C5aR. The results showed that exposure of neutrophils to acapsular cryptococci produced up-regulation of C5aR expression; in contrast, exposure to encapsulated cryptococci or acapsular cryptococci plus purified GXM produced down-regulation.
MATERIALS AND METHODS
Reagents and media.
GXM was isolated from culture supernatant fluid of a serotype A strain (ATCC 24064) that had been grown in liquid synthetic medium (22) in a gyratory shaker for 4 days at 30°C. GXM was isolated by differential precipitation with ethanol and hexadecyl trimethyl ammonium bromide (5, 6).
Rabbit anti-human CD88 (C5aR) immunoglobulin G (IgG) was purchased from Serotec Ltd. (Oxford, England). A murine monoclonal antibody (MAb) (IgG1 isotype) that is reactive with human CD18 was obtained from Monosan (Uden, The Netherlands). Fluorescein isothiocyanate (FITC)-conjugated murine anti-rabbit MAbs (IgG1 isotype), F(ab′)2 fragments of R-phycoerythrin-conjugated sheep anti-mouse IgG (whole molecule), FITC-conjugated murine IgG1 from murine myeloma (isotype control), and purified rabbit IgG (irrelevant antibody) were purchased from Sigma (St. Louis, Mo.).
Human C5a was purchased from Calbiochem-Novabiochem Corporation (La Jolla, Calif.). Hemacolor was obtained from Merck (Merck KgaA, Darmstadt, Germany). RPMI 1640 with glutamine and fetal calf serum (FCS) were obtained from Gibco BRL (Paisley, Scotland). HS type AB was purchased from Sigma. All reagents and media used in this study were negative for endotoxin, as detected by a Limulus amebocyte lysate assay (Sigma), which had a sensitivity of approximately 0.05 to 0.1 ng of Escherichia coli lipopolysaccharide per ml.
Microorganisms.
Two strains of C. neoformans var. neoformans were obtained from Centraalbureau voor Schimmelcultures (Delft, The Netherlands): a thinly encapsulated strain of serotype A (6995; NIH 37) and an acapsular strain (7698; NIH B-4131). The cultures were maintained by serial passages on Sabouraud agar (Biomerieux, Lyon, France). Log-phase yeasts were harvested by suspending a single colony in RPMI 1640 and then were washed twice with saline, counted with a hemocytometer, and adjusted to the desired concentration in RPMI 1640 (44). Yeasts were used live or were heat killed at 60°C for 30 min.
Preparation of PMN.
Heparinized venous blood obtained from healthy adult blood donors ranging in age from 18 to 65 years (recruited from the Immunohematology and Transfusion Service of the Policlinico Hospital, Perugia, Italy) was diluted with RPMI 1640, and mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation (34). The pellet containing PMN and erythrocytes was treated with hypotonic saline to lyse the erythrocytes. Granulocytes were collected by centrifugation, washed twice with RPMI 1640, counted, and adjusted to the desired concentration. The purity of PMN isolated by this method was >98%, as determined by Giemsa staining.
Surface expression of C5aR.
PMN (106) were preincubated for 30 min in RPMI 1640 with 10% HS or 10% heat-inactivated (56°C for 30 min) HS (iHS) and with or without 7698 cells (effector cell/target cell [E/T] ratio, 1:4), 6995 cells (E/T ratio, 1:4), GXM alone (250 μg/ml), or 7698 cells (E/T ratio, 1:4) pretreated with GXM (250 μg/ml for 30 min at 37°C). The PMN were then fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature, washed twice with PBS containing 0.5% bovine serum albumin and 0.1% sodium azide, and mixed with rabbit anti-human C5aR (1/100). After 30 min of incubation on ice, the cells were washed twice and then stained for 30 min with an FITC-conjugated murine MAb specific for rabbit IgG or an FITC-conjugated antibody isotype control (final dilution, 1/256). For each sample, C5aR expression on the surface of CD18-positive cells was measured by using fluorescence-activated cell sorting (Becton Dickinson, Mountain View, Calif.). PMN were further distinguished from free yeast cells on the basis of forward and side light scattering, and a discriminatory gate was placed around the PMN cluster. Specific fluorescence was assessed by comparison with results obtained with an irrelevant MAb that was isotype matched for the FITC-conjugated murine MAb that was reactive with rabbit IgG. Autofluorescence was assessed by using unstained cells.
Binding of FLUOS-C5a to PMN.
C5a was labeled with 5(6)-carboxyfluorescein-N-hydroxysuccinimide ester (FLUOS) by use of a labeling kit according to the manufacturer's directions (Boehringer Mannheim Biochimica, Mannheim, Germany). Binding of FLUOS-C5a to PMN was determined as described previously (40). Briefly, PMN were incubated with FLUOS-C5a (25 nM) for 20 min at room temperature. The cells were washed twice with PBS containing 0.5% bovine serum albumin and 0.1% sodium azide and suspended in 0.5 ml of PBS containing 0.5% bovine serum albumin and 0.1% sodium azide. Binding was assessed by flow cytometry.
Superoxide (O2−) production.
O2− production by PMN in response to stimulation with C5a was determined by superoxide dismutase-inhibitable cytochrome c reduction as described previously (34). The effect of various treatments on the response of PMN to C5a was determined by first treating PMN (106) for 30 min at 37°C in RPMI 1640, RPMI 1640 with 10% iHS, or RPMI 1640 with 10% HS and 6995 cells (E/T ratio, 1/4), 7698 cells (E/T ratio, 1/4), GXM alone (250 μg/ml), or 7698 cells pretreated with GXM (250 μg/ml for 30 min at 37°C). The variously treated PMN (2.5 × 105) were washed and incubated in Krebs-Ringer phosphate buffer with or without C5a (50 ng/ml) for 30 min at 37°C in the presence of cytochrome c (2 mg/ml; Sigma) and in the presence or absence of superoxide dismutase (200 U/ml; Sigma). The reaction was stopped by the addition of 2 ml of cold Krebs-Ringer phosphate buffer, and the tubes were centrifuged at 2,000 × g for 5 min at 25°C. The amount of superoxide in the supernatant fluid was measured as the absorbance of superoxide-dependent cytochrome c reduction by using an extinction coefficient of 21.0 × 103 M/cm at 550 nm. Superoxide production was expressed in nanomoles per 106 cells for 30 min.
Chemotaxis assay.
A PMN chemotaxis assay was performed as described previously (11). Briefly, we used a 48-well microchemotactic Boyden chamber (NeuroProbe Inc., Bethesda, Md.) with a 10-μm-thick, polyvinylpyrrolidone-free polycarbonate membrane (Nuclepore Corp., Pleasanton, Calif.) with a 3-μm pore size. A polyvinylpyrrolidone-free polycarbonate membrane was used because PMN remain attached to the membrane and do not drop off into the medium below (19). PMN (1.5 × 106/ml) were preincubated for 30 min at 37°C with 6995 cells (E/T ratio, 1:4), 7698 cells (E/T ratio, 1:4), GXM alone (250 μg/ml), or 7698 cells pretreated with GXM (250 μg/ml for 30 min at 37°C) in RPMI 1640 plus 10% HS. The PMN were then washed twice and suspended in RPMI 1640 plus 1% HS in accordance with the chamber manufacturer's directions. The chamber, with 50 μl of PMN (1.5 × 106/ml) in RPMI 1640 plus 1% HS in the upper compartment and 27 μl of C5a (50 ng/ml) in the lower compartment, was incubated for 60 min at 37°C. The membrane was stained with Hemacolor. The numbers of PMN that migrated to the reverse side of the membrane were counted in three high-power microscopic fields. The results are reported as the mean and standard error of triplicate determinations from three separate experiments.
Statistical analysis.
Statistical analysis was performed with the assistance of the Primer of Biostatistics software program. Data are reported as the mean and standard error of the mean from replicate experiments. Data were evaluated by a one-way analysis of variance (ANOVA). Post hoc comparisons were done with Bonferroni's test. A P value of <0.05 was taken as significant.
RESULTS
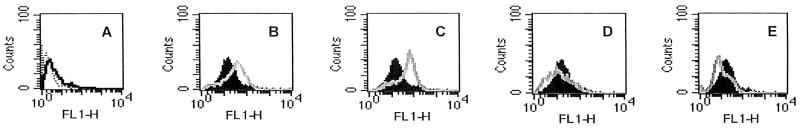
To assess the effect of exposure to cryptococci on C5aR expression, human neutrophils were incubated with (i) heat-killed or live acapsular cryptococci or (ii) heat-killed or live encapsulated cryptococci. The results showed that there was clear up-regulation of C5aR expression on neutrophils exposed to heat-inactivated or live acapsular C. neoformans (Fig. 1B and C). In contrast, the suppression of C5aR expression was observed when neutrophils were treated with heat-killed or live encapsulated cells (Fig. 1D and E).
FIG. 1.
Cytofluorimetric analysis of C5aR expression on PMN. PMN were incubated for 30 min in the absence (black histograms) or in the presence (grey lines) of (i) heat-killed acapsular cryptococci (7698) (E/T ratio, 1:4) (B), (ii) live 7698 (E/T ratio, 1:4) (C), (iii) heat-killed encapsulated cryptococci (6995) (E/T ratio, 1:4) (D), or (iv) live 6995 (E/T ratio, 1:4) (E). (A) Control showing autofluorescence of the cells (dotted line) and staining profile with an irrelevant antibody (black line). The results are from one experiment representative of four with similar results, performed by using cells from four different healthy subjects. FL1-H, fluorescent channel 1 height.
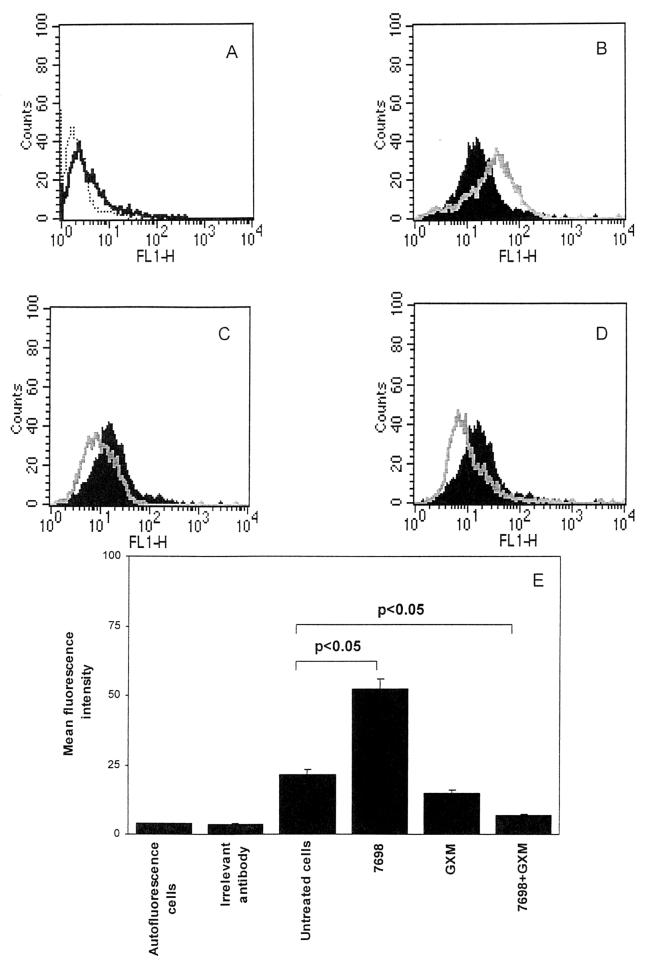
The down-regulation of C5aR expression by encapsulated cryptococci suggested that the major capsular polysaccharide, GXM, might play a role in the suppression phenomenon. Consequently, we evaluated the effect of GXM, alone or in combination with cells of the acapsular strain, on neutrophil C5aR expression. Preincubation of acapsular cryptococci with GXM was examined because previous studies found that GXM binds to acapsular cryptococci to produce an experimentally constructed capsule that behaves functionally like that found on encapsulated cryptococci (25, 28). The results confirmed that acapsular cryptococci up-regulated C5aR expression (Fig. 2B). In contrast, GXM (250 μg/ml) alone produced a slight inhibition of constitutive C5aR expression (Fig. 2C) that was not statistically significant (Fig. 2E). Dose-response experiments showed that GXM at 10 or 50 μg/ml was unable to reduce C5aR expression (data not shown). GXM (250 μg/ml) used in combination with acapsular cryptococci decreased C5aR expression (Fig. 2D) relative to both the enhanced expression observed with acapsular cryptococci alone and the constitutive expression of C5aR observed with unstimulated PMN. In a dose-response experiment, the addition of GXM to acapsular cryptococci produced a significant down-regulation of C5aR expression at 50 μg/ml but not at 10 μg/ml (data not shown).
FIG. 2.
Cytofluorimetric analysis of C5aR expression on PMN with GXM. PMN were incubated for 30 min in the absence (black histograms) or in the presence (grey lines) of (i) heat-killed acapsular cryptococci (7698) (E/T ratio, 1:4) (B), (ii) GXM alone (250 μg/ml) (C), or (iii) 7698 cells (E/T ratio, 1:4) pretreated with GXM (D). (A) Control showing autofluorescence of the cells (dotted line) and staining profile with an irrelevant antibody (black line). The results are from one experiment representative of four with similar results, performed by using cells from four different healthy subjects. (E) Mean fluorescence intensity for all groups. The results are the mean and standard error of the mean for four independent experiments with cells from four different healthy subjects. Statistical significance was determined by using ANOVA with Bonferroni's posttest analysis. FL1-H, fluorescent channel 1 height.
There are two alternative explanations for some of the results shown in Fig. 1 and 2. First, soluble GXM could have been washed off the encapsulated cells, and free GXM could have influenced the results. This possibility was examined by incubating encapsulated cryptococci with PMN for 30 min at 37°C in 10% HS. The supernatant fluid was collected and mixed with neutrophils, and C5aR expression was determined after 30 min of incubation. The results showed no modulation of C5aR by the supernatant fluid (data not shown). A second explanation for the modulation of C5aR expression is the possibility that the effect was due to the binding of C3b or iC3b to the yeast surface during incubation with 10% HS. Alternatively, the effect could have been due to the release of C3a or C5a as a consequence of activation of the alternative pathway. These possibilities were assessed by use of heat-inactivated serum (56°C for 30 min) in place of normal serum. The results of experiments done with heat-inactivated serum were similar to the results of experiments done in the presence of normal serum (data not shown).
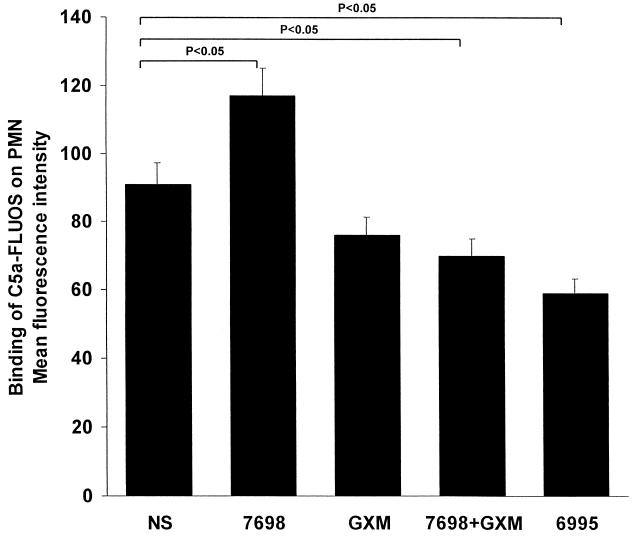
Having determined that acapsular and encapsulated cryptococci differentially regulate C5aR expression, we next examined whether such differential regulation of C5aR expression influences the binding of C5a to neutrophils. PMN were incubated in the presence or absence of GXM alone (250 μg/ml), acapsular yeast cells alone, acapsular yeast cells in combination with GXM, or encapsulated yeast cells alone. After 30 min, the cells were mixed with FLUOS-C5a, incubated for 20 min, and washed. The binding of FLUOS-C5a was determined by flow cytometry. The results (Fig. 3) showed enhanced binding of C5a to PMN that were stimulated with acapsular cryptococci. Significantly decreased binding (P < 0.05) occurred when PMN were pretreated with encapsulated cryptococci or acapsular cryptococci plus GXM. Preincubation of PMN with GXM alone produced a slight reduction in the binding of C5a; this binding was not significantly (P < 0.05) lower than the binding to sham-treated PMN.
FIG. 3.
Cytofluorimetric analysis of C5a binding on PMN. PMN were untreated (NS) or treated with heat-killed acapsular cryptococci (7698) (E/T ratio, 1:4), GXM alone (250 μg/ml), heat-killed 7698 (E/T ratio, 1:4) pretreated with GXM, or heat-killed encapsulated cryptococci (6995) (E/T ratio, 1:4). The results are the mean and standard error of the mean for three independent experiments with cells from three different healthy subjects. Statistical significance was determined by using ANOVA with Bonferroni's posttest analysis.
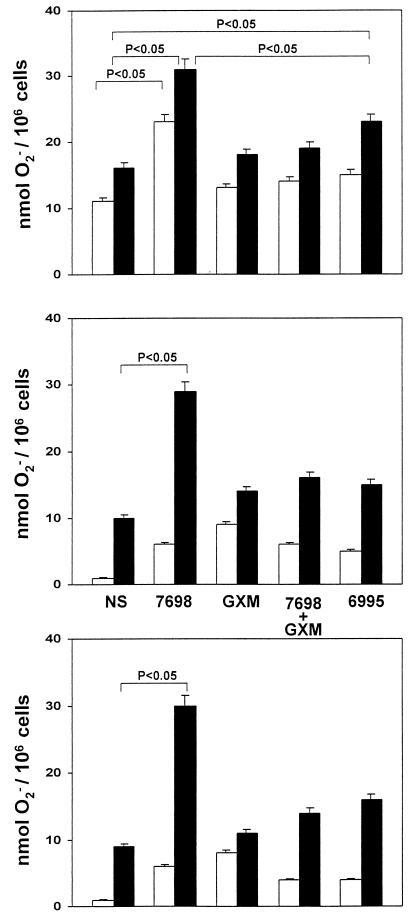
Given that C5a ligation on PMN may produce significant biological effects, such as superoxide anion generation (30), we evaluated the production of O2− in response to stimulation with C5a of PMN treated with (i) acapsular cryptococci, (ii) GXM alone, (iii) acapsular cryptococci plus GXM, or (iv) encapsulated cryptococci. PMN were incubated for 30 min with each yeast treatment, C5a was added, and the mixtures were incubated for 30 min. Since incubation of acapsular or encapsulated cryptococci in 10% serum will likely generate C5a through the action of the alternative pathway, the experiment was done in the presence of normal serum, in the presence of heat-inactivated serum, and in the absence of serum. The results (Fig. 4) showed that treatment of PMN with acapsular cryptococci produced a significant (P < 0.05) enhancement of superoxide generation in response to stimulation with C5a compared to sham treatment of PMN. This enhancement occurred in the presence of normal serum, in the presence of heat-inactivated serum, and in the absence of serum. Treatment with GXM alone or acapsular cells plus GXM produced a slight increase in O2− generation that was not significantly different from the response of sham-treated PMN. Notably, the level of O2− generation by PMN treated with acapsular cells plus GXM was significantly lower than the level of O2− generation by PMN treated with acapsular cells alone. Surprisingly, treatment with encapsulated cryptococci produced a significant increase in O2− production in response to C5a relative to what was observed with sham-treated PMN.
FIG. 4.
Superoxide-generating activity of PMN in response to C5a (50 ng/ml) after 30 min of incubation. PMN were untreated (NS) or pretreated for 30 min with heat-killed acapsular cryptococci (7698) (E/T ratio, 1:4), GXM alone (250 μg/ml), heat-killed 7698 (E/T ratio, 1:4) pretreated with GXM, or heat-killed encapsulated cryptococci (6995) (E/T ratio, 1:4). C5a was absent (white bars) or present (black bars). The results are from cells incubated in the presence of normal HS (top) or iHS (middle) or in the absence of HS (bottom). The results are the mean and standard error of the mean for six independent experiments with cells from six different healthy subjects. Statistical significance was determined by using ANOVA with Bonferroni's posttest analysis.
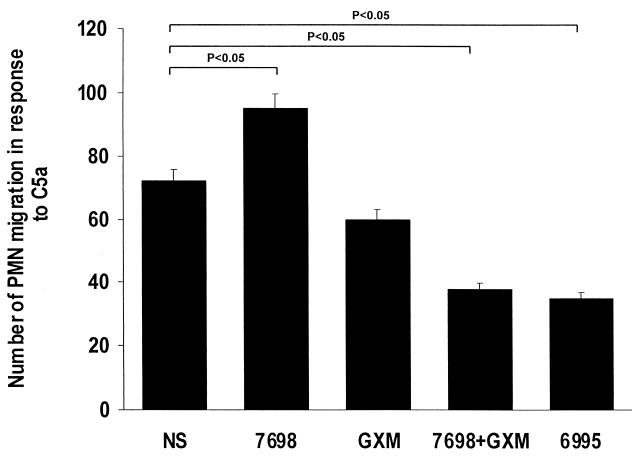
The ability to produce chemotactic migration of neutrophils is one of the most important inflammatory properties of C5a. As a consequence, we determined the effect of pretreatment of neutrophils with cryptococci or GXM on the chemotactic response to C5a. The results (Fig. 5) showed that the migration of PMN toward C5a was significantly enhanced by pretreatment with acapsular cryptococci. In contrast, treatment with encapsulated cryptococci or acapsular cryptococci plus GXM produced a significant decrease in migration. Treatment of PMN with GXM alone produced a slight decrease in migration that was not significantly different from what was observed with sham-treated PMN.
FIG. 5.
Chemotactic responses of PMN to C5a (50 ng/ml). PMN were incubated for 30 min at 37°C in 5% CO2 alone (NS) or with heat-killed acapsular cryptococci (7698) (E/T ratio, 1:4), GXM alone (250 μg/ml), heat-killed 7698 (E/T ratio, 1:4) pretreated with GXM, or heat-killed encapsulated cryptococci (6995) (E/T ratio, 1:4). The results are the mean and standard error of the mean for three independent experiments with cells from three different healthy subjects. Statistical significance was determined by using ANOVA with Bonferroni's posttest analysis.
DISCUSSION
The results reported here show that treatment of PMN with C. neoformans modulates the expression of C5aR. Treatment of PMN with acapsular cryptococci up-regulated receptor expression, whereas treatment with encapsulated cryptococci down-regulated expression. Down-regulation by encapsulated cryptococci was due to encapsulation with GXM, because we found that the increased expression in response to acapsular cryptococci reverted to the suppressed expression found with encapsulated cells when purified GXM was added to the milieu. Previous studies demonstrated that incubation of acapsular cryptococci with GXM leads to the binding of GXM to the acapsular cells to produce cells that are functionally encapsulated (25, 28).
The modulation of receptor expression had functional consequences. PMN treated with acapsular cryptococci showed increased binding of C5a, increased production of superoxide in response to stimulation with C5a, and increased migration in response to C5a. In contrast, treatment of PMN with encapsulated cryptococci or acapsular cryptococci plus GXM down-regulated receptor expression and suppressed migration in response to C5a. Suppression of superoxide production was not observed after treatment with encapsulated cryptococci or acapsular cryptococci plus GXM. Indeed, treatment with encapsulated cryptococci produced a slight increase in superoxide production, suggesting that encapsulated cryptococci might have effects on superoxide production by PMN that are independent of the effects on C5aR expression. The increased responsiveness to C5a was not due to generation of C5a from serum by activation of the complement cascade, because the slight increase in responsiveness was also observed when the assay was done in the presence of heat-inactivated serum or in the absence of serum.
The effects of GXM on C5aR expression and the functional consequences of such effects appeared to be primarily associated with encapsulation. Purified GXM alone had a modest direct down-regulatory effect on receptor expression and slight effects on binding of C5a and the chemotactic response to C5a. However, these latter effects were not statistically significant. There is precedent for GXM to display biological activities when assembled in the form of a capsule, but it fails to show the same biological activity when in the soluble form. For example, the cryptococcal capsule is a potent activator of the complement system, and the amounts of C3 fragments that accumulate on yeast cells are directly related to capsule volume (45); however, purified GXM has little or no ability to activate the alternative pathway (31). In a second example, encapsulated cryptococci stimulate the lymphoproliferative response of T cells to Cryptococcus-laden monocytes and CD40 expression by monocytes; in contrast, soluble GXM stimulates CD40 expression but does not induce lymphoproliferation (43).
One possible explanation for the reduced binding of C5a is masking of the binding of C5a or an antibody reactive with C5aR by GXM. This scenario might be possible with free GXM, but the inhibitory effect of encapsulated cryptococci was greater than the effect observed with free GXM. More importantly, the suppression of C5aR expression was observed with encapsulated cryptococci at an E/T ratio of 1:4. Masking of the receptor would require binding to all possible receptors. This scenario seems highly unlikely at an E/T ratio of 1:4, because a PMN is much larger than a yeast cell. Our results are much more consistent with a signaling phenomenon.
Previous studies demonstrated the biological importance of C5aR. It belongs to a family of G proteins that regulate the activation and trafficking of phagocytic cells and include various chemokine receptors and the receptors for platelet-activating factor and proinflammatory leukotrienes. C5aR mediates proinflammatory and chemotactic actions, granule enzyme release, and superoxide anion production. C5aR has been implicated in anaphylactic and septic shock (16, 18, 35, 38). The biological importance of C5aR expression in infectious diseases is indirectly suggested by the ability of several microbial pathogens to destroy this receptor and/or its ligand, C5a, via a variety of proteinases (24). In addition,C5aR-knockout mice are exquisitely susceptible to pulmonary infection by Pseudomonas aeruginosa, in a pathologic process resembling human cystic fibrosis (21).
The cryptococcal capsule and/or its major capsular polysaccharide mediate two opposing biological activities. On the one hand, the cryptococcal capsule is a powerful activator of the complement system (26, 27). Incubation of encapsulated cells in HS leads to the generation of cleavage fragments of the complement cascade that would normally mediate a potent inflammatory response to infection. In addition, Dong and Murphy found that a yeast culture filtrate stimulated the directed migration of PMN in the absence of serum (11). The direct or indirect production of inflammatory mediators in response to C. neoformans is in striking contrast to the relative absence of neutrophil infiltration in most infected tissues (3). Several activities of C. neoformans or cryptococcal antigens have been described that may contribute to the relative absence of PMN infiltration. First, GXM induces neutrophils to shed L-selectin, a molecule that is needed for the infiltration of neutrophils into tissue (12). Second, GXM binds to adhesion molecule CD18 (13). Since an interaction between CD18 and its ligand on the endothelium is a prerequisite for tissue infiltration, CD18 blockade may also contribute to the absence of PMN infiltration in infected tissues. Our results showing direct down-regulation of C5aR expression by GXM in the form of a cryptococcal capsule and the consequent suppression of biological activities associated with C5aR ligation are a third mechanism by which cryptococci may evade the inflammatory response that would normally be expected to accompany activation of the complement cascade by the cryptococcal capsule. The down-regulatory effects of encapsulated cryptococci on C5aR activity most likely occur at the site of infection, because circulating PMN rarely encounter encapsulated cells. However, at the site of infection, PMN should encounter encapsulated cryptococci, with a consequent modulation of C5aR and an accompanying modulation of PMN biological function, such as superoxide generation. It is also possible that PMN with reduced C5aR activity fail to become activated in response to C5a and exhibit reduced phagocytosis and/or killing of cryptococci. Taken together, our results, although limited to in vitro observations, suggest an additional mechanism for immunoevasion produced by cryptococcal GXM when it is presented in the form of a capsule.
Acknowledgments
We thank Jo-Anne Rowe for excellent secretarial and editorial support.
This study was supported by a grant from the National Research Program on AIDS (Opportunistic Infections and Tuberculosis, contract 50D.31), Rome, Italy, and by U.S. Public Health Service grant AI-14209 (to T.R.K.).
Editor: R. N. Moore
REFERENCES
- 1.Byron, J. K., K. V. Clemons, J. H. McCusker, R. W. Davis, and D. A. Stevens. 1995. Pathogenicity of Saccharomyces cerevisiae in complement factor five-deficient mice. Infect. Immun. 63:478-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calich, V. L., T. L. Kipnis, M. Mariano, C. F. Neto, and W. D. Dias da Silva. 1979. The activation of the complement system by Paracoccidioides brasiliensis in vitro: its opsonic effect and possible significance for an in vivo model of infection. Clin. Immunol. Immunopathol. 12:21-30. [DOI] [PubMed] [Google Scholar]
- 3.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans, p. 177-222. American Society for Microbiology, Washington, D.C.
- 4.Cassatella, M. A. 1995. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today 16:21-26. [DOI] [PubMed] [Google Scholar]
- 5.Cherniak, R., E. Reiss, M. E. Slodki, R. D. Plattner, and S. O. Blumer. 1980. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans. Mol. Immunol. 17:1025-1032. [DOI] [PubMed] [Google Scholar]
- 6.Cherniak, R., E. Reiss, and S. H. Turner. 1982. A galactoxylomannan antigen of Cryptococcus neoformans serotype A. Carbohydr. Res. 103:239-250. [Google Scholar]
- 7.Crass, T., R. S. Ames, H. M. Sarau, M. A. Tornetta, J. J. Foley, J. Köhl, A. Klos, and W. Bautsch. 1999. Chimeric receptors of the human C3a receptor and C5a receptor (CD88). J. Biol. Chem. 274:8367-8370. [DOI] [PubMed] [Google Scholar]
- 8.Czermak, B. J., A. B. Lentsch, N. M. Bless, H. Schmal, H. P. Friedl, and P. A. Ward. 1998. Role of complement in in vitro and in vivo lung inflammatory reactions. J. Leukoc. Biol. 64:40-48. [DOI] [PubMed] [Google Scholar]
- 9.Diamond, R. D., and N. F. Erickson III. 1982. Chemotaxis of human neutrophils and monocytes induced by Cryptococcus neoformans. Infect. Immun. 38:380-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamond, R. D., J. E. May, M. Kane, M. M. Frank, and J. E. Bennett. 1973. The role of late complement components and the alternate complement pathway in experimental cryptococcosis. Proc. Soc. Exp. Biol. Med. 144:312-315. [DOI] [PubMed] [Google Scholar]
- 11.Dong, Z. M., and J. W. Murphy. 1993. Mobility of human neutrophils in response to Cryptococcus neoformans cells, culture filtrate antigen, and individual components of the antigen. Infect. Immun. 61:5067-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong, Z. M., and J. W. Murphy. 1996. Cryptococcal polysaccharides induce l-selectin shedding and tumor necrosis factor receptor loss from the surface of human neutrophils. J. Clin. Investig. 97:689-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong, Z. M., and J. W. Murphy. 1997. Cryptococcal polysaccharide binds to CD18 on human neutrophils. Infect. Immun. 65:557-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer, W. H., M. A. Jagels, and T. E. Hugli. 1999. Regulation of IL-6 synthesis in human peripheral blood mononuclear cells by C3a and C3a (desArg). J. Immunol. 162:453-459. [PubMed] [Google Scholar]
- 15.Gelfand, J. A., D. L. Hurley, A. S. Fauci, and M. M. Frank. 1978. Role of complement in host defense against experimental disseminated candidiasis. J. Infect. Dis. 138:9-16. [DOI] [PubMed] [Google Scholar]
- 16.Gerard, N. P., and C. Gerard. 1991. The chemotactic receptor for human C5a anaphylatoxin. Nature 349:614-617. [DOI] [PubMed] [Google Scholar]
- 17.Graybill, J. R., and J. Ahrens. 1981. Immunization and complement interaction in host defense against murine cryptococcosis. J. Reticuloendothel. Soc. 30:347-357. [PubMed] [Google Scholar]
- 18.Hack, C. E., J. H. Nuijens, R. J. Felt-Bersma, W. O. Schreuder, A. J. Eerenberg-Belmer, J. Paardekooper, W. Bronsveld, and L. G. Thijs. 1989. Elevated plasma levels of the anaphylatoxins C3a and C4a are associated with a fatal outcome in sepsis. Am. J. Med. 86:20-26. [DOI] [PubMed] [Google Scholar]
- 19.Harvath, L., W. Falk, and E. J. Leonard. 1980. Rapid quantitation of neutrophil chemotaxis: use of a polyvinylpyrrolidone-free polycarbonate membrane in a multiwell assembly. J. Immunol. Methods 37:39-45. [DOI] [PubMed] [Google Scholar]
- 20.Hector, R. F., E. Yee, and M. S. Collins. 1990. Use of DBA/2N mice in models of systemic candidiasis and pulmonary and systemic aspergillosis. Infect. Immun. 58:1476-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopken, U. E., B. Lu, N. P. Gerard, and C. Gerard. 1996. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature 383:86-89. [DOI] [PubMed] [Google Scholar]
- 22.Houpt, D. C., G. S. T. Pfrommer, B. J. Young, T. A. Larson, and T. R. Kozel. 1994. Occurrences, immunoglobulin classes, and biological activities of antibodies in normal human serum that are reactive with Cryptococcus neoformans glucuronoxylomannan. Infect. Immun. 62:2857-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huey, R., and T. E. Hugli. 1985. Characterization of a C5a receptor on human polymorphonuclear leukocytes (PMN). J. Immunol. 135:2063-2068. [PubMed] [Google Scholar]
- 24.Jagels, M. A., J. A. Ember, J. Travis, J. Potempa, R. Pike, and T. E. Hugli. 1996. Cleavage of the human C5a receptor by proteinases derived from Porphyromonas gingivalis: cleavage of leukocytes C5a receptor. Adv. Exp. Med. Biol. 389:155-164. [DOI] [PubMed] [Google Scholar]
- 25.Kozel, T. R. 1977. Nonencapsulated variant of Cryptococcus neoformans. II. Surface receptors for cryptococcal polysaccharide and their role in inhibition of phagocytosis by polysaccharide. Infect. Immun. 16:99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozel, T. R. 1996. Activation of the complement system by pathogenic fungi. Clin. Microbiol. Rev. 9:34-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozel, T. R. 1998. Complement activation by pathogenic fungi. Res. Immunol. 149:309-320. [DOI] [PubMed] [Google Scholar]
- 28.Kozel, T. R., and C. A. Hermerath. 1984. Binding of cryptococcal polysaccharide to Cryptococcus neoformans. Infect. Immun. 43:879-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozel, T. R., and G. S. Pfrommer. 1986. Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect. Immun. 52:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozel, T. R., M. A. Wilson, G. S. Pfrommer, and A. M. Schlageter. 1989. Activation and binding of opsonic fragments of C3 on encapsulated Cryptococcus neoformans by using an alternative complement pathway reconstituted from six isolated proteins. Infect. Immun. 57:1922-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laxalt, K. A., and T. R. Kozel. 1979. Chemotoxigenesis and activation of the alternative complement pathway by encapsulated and nonencapsulated Cryptococcus neoformans. Infect. Immun. 26:435-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovchik, J. A., and M. F. Lipscomb. 1993. Role of C5 and neutrophils in the pulmonary intravascular clearance of circulating Cryptococcus neoformans. J. Respir. Cell. Mol. Biol. 9:617-627. [DOI] [PubMed] [Google Scholar]
- 33.Monari, C., A. Casadevall, D. Pietrella, F. Bistoni, and A. Vecchiarelli. 1999. Neutrophils from patients with advanced HIV infection have impaired complement receptor function and preserved Fcγ receptor function. J. Infect. Dis. 180:1542-1549. [DOI] [PubMed] [Google Scholar]
- 34.Monari, C., A. Casadevall, C. Retini, F. Baldelli, F. Bistoni, and A. Vecchiarelli. 1999. Antibody to capsular polysaccharide enhances the function of neutrophils from patients with AIDS against Cryptococcus neoformans. AIDS 13:653-660. [DOI] [PubMed] [Google Scholar]
- 35.Nosanchuk, J. D., and A. Casadevall. 1997. Cellular charge of Cryptococcus neoformans: contributions from the capsular polysaccharide, melanin, and monoclonal antibody binding. Infect. Immun. 65:1836-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pellas, T. C., W. Boyar, J. van Oostrum, J. Wasvary, L. R. Fryer, G. Pastor, M. Sills, A. Braunwalder, D. R. Yarwood, R. Kramer, E. Kimble, J. Hadala, W. Haston, R. Moreira-Ludewig, S. Vziel-Fusi, P. Peters, K. Bill, and L. P. Wennogle. 1998. Novel C5a receptor antagonists regulate neutrophil functions in vitro and in vivo. J. Immunol. 160:5616-5621. [PubMed] [Google Scholar]
- 37.Retini, C., A. Vecchiarelli, C. Monari, C. Tascini, F. Bistoni, and T. R. Kozel. 1996. Capsular polysaccharide of Cryptococcus neoformans induces proinflammatory cytokine release by human neutrophils. Infect. Immun. 64:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shuster, D. E., M. E. Kehrli, P. Rainard, and M. Paape. 1997. Complement fragment C5a and inflammatory cytokines in neutrophil recruitment during intramammary infection with Escherichia coli. Infect. Immun. 65:3286-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van den Berg, C. W., P. C. Aerts, and H. Van Dijk. 1991. In vivo anti-complementary activities of the cobra venom factors from Naja naja and Naja haje. J. Immunol. Methods 136:287-294. [DOI] [PubMed] [Google Scholar]
- 40.Van Epps, D. E., and D. E. Chenoweth. 1984. Analysis of the binding of fluorescent C5a and C3a to human peripheral blood leukocytes. J. Immunol. 132:2862-2867. [PubMed] [Google Scholar]
- 41.Vecchiarelli, A., C. Monari, B. Palazzetti, F. Bistoni, and A. Casadevall. 2000. Dysregulation in IL-12 secretion by neutrophils from HIV-infected patients. Clin. Exp. Immunol. 121:311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vecchiarelli, A., C. Retini, A. Casadevall., C. Monari, D. Pietrella, and T. R. Kozel. 1998. Involvement of C3a and C5a in interleukin-8 secretion by human polymorphonuclear cells in response to capsular material of Cryptococcus neoformans. Infect. Immun. 66:4324-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vecchiarelli, A., C. Retini, D. Pietrella, C. Monari, and T. R. Kozel. 2000. T lymphocyte interaction by CD40/CD40 ligand facilitates a lymphoproliferative response and killing of Cryptococcus neoformans in vitro. Eur. J. Immunol. 30:1385-1393. [DOI] [PubMed] [Google Scholar]
- 44.Vecchiarelli, A., C. Retini, D. Pietrella, C. Monari, C. Tascini, T. Beccari, and T. R. Kozel. 1995. Down-regulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1β secretion from human monocytes. Infect. Immun. 63:2919-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young, B. J., and T. R. Kozel. 1993. Effects of strain variation, serotype, and structural modification on kinetics for activation and binding of C3 to Cryptococcus neoformans. Infect. Immun. 61:2966-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]