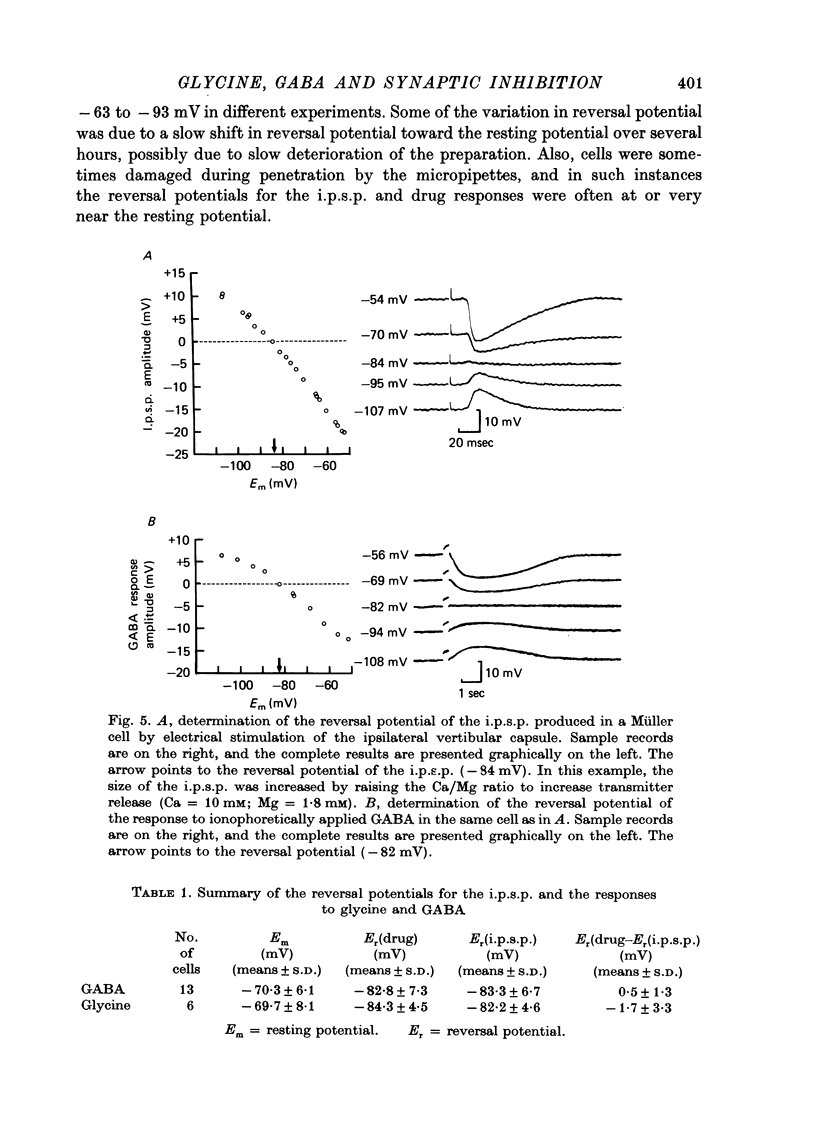
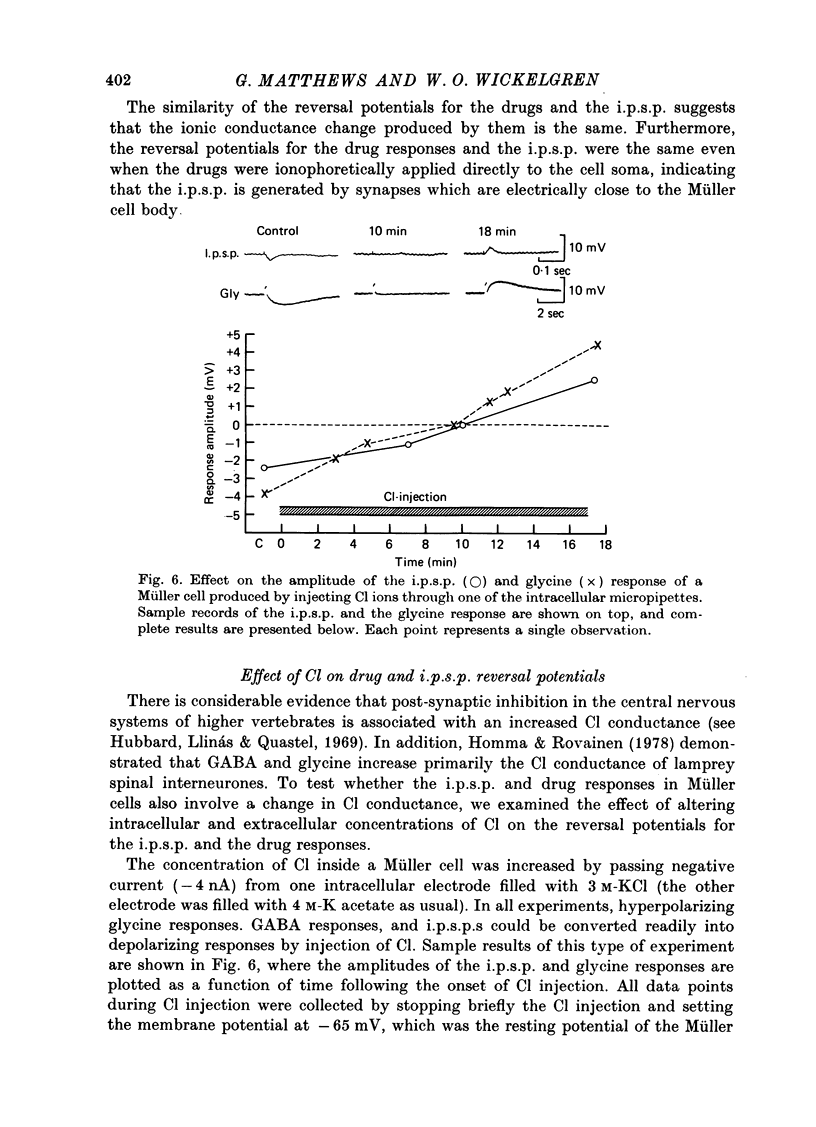
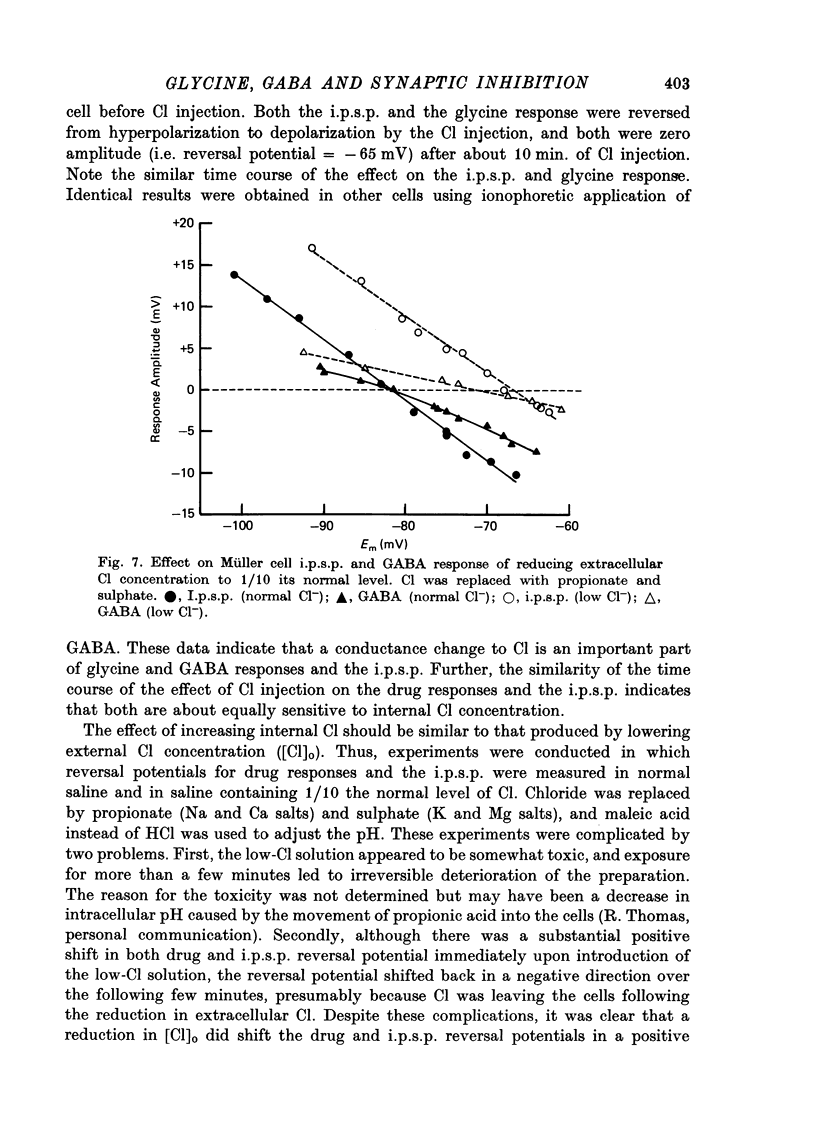
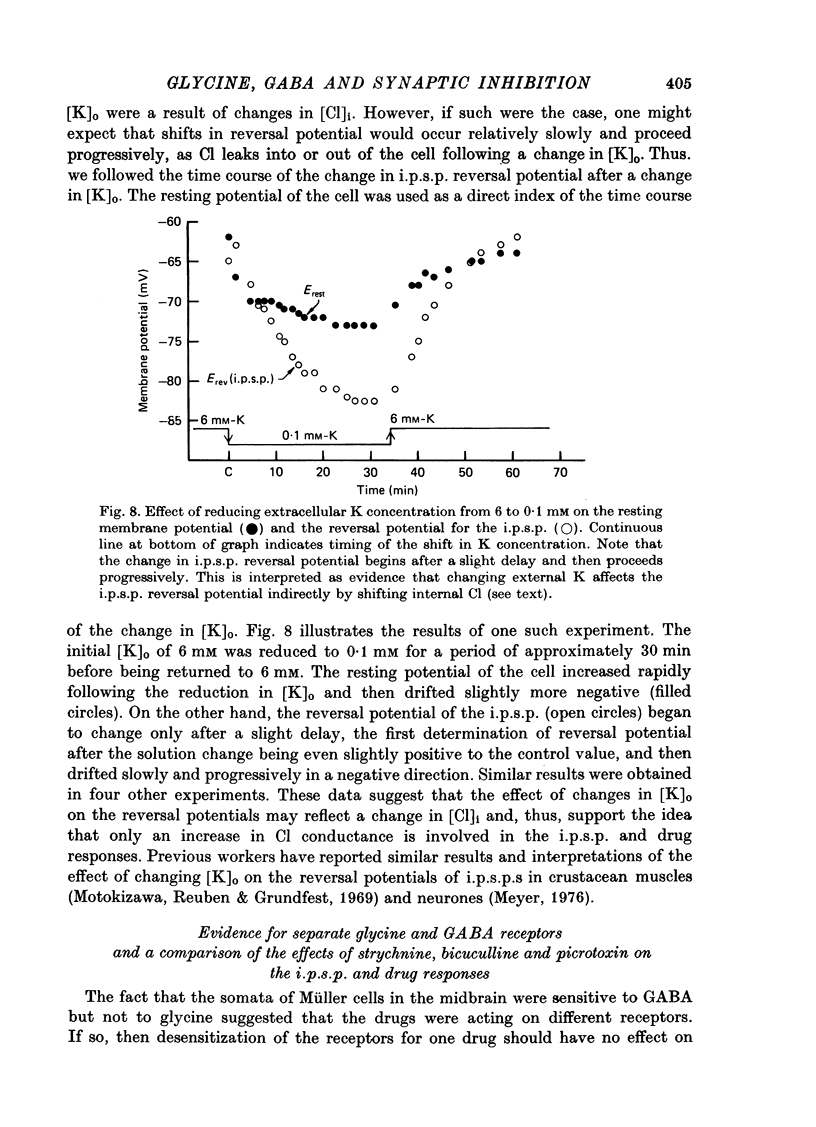
Abstract
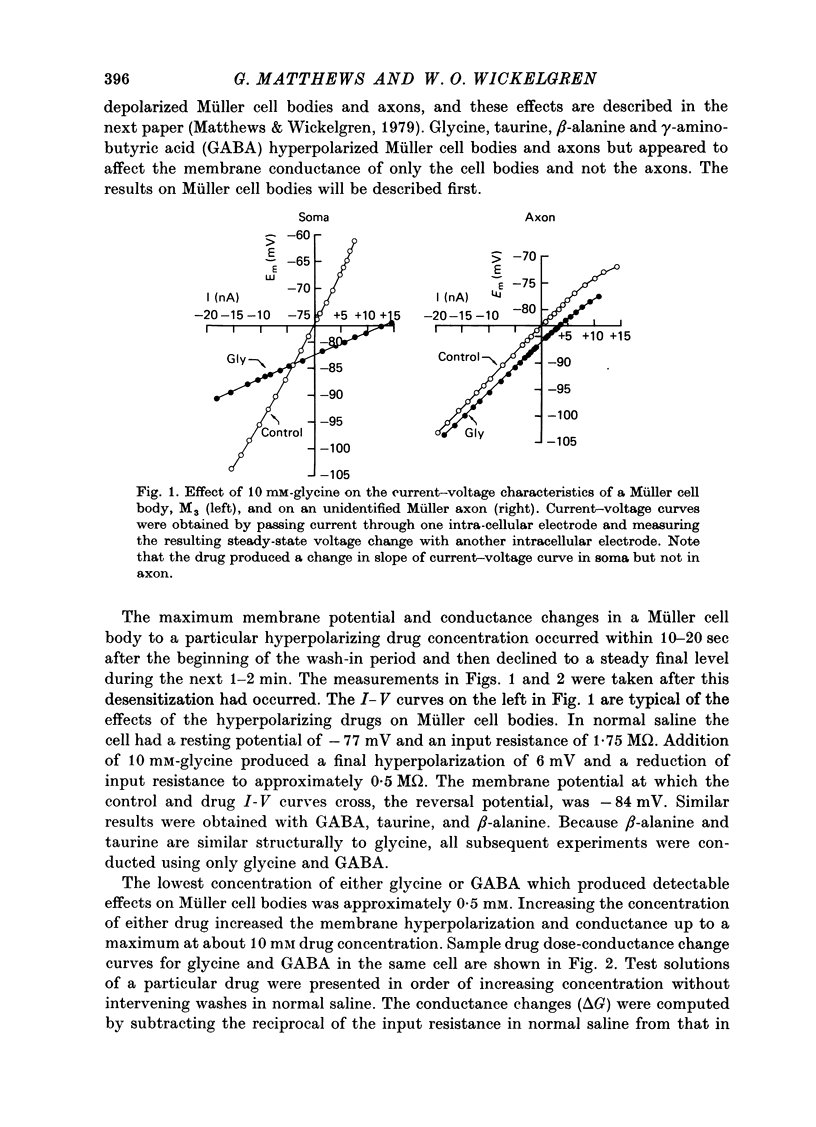
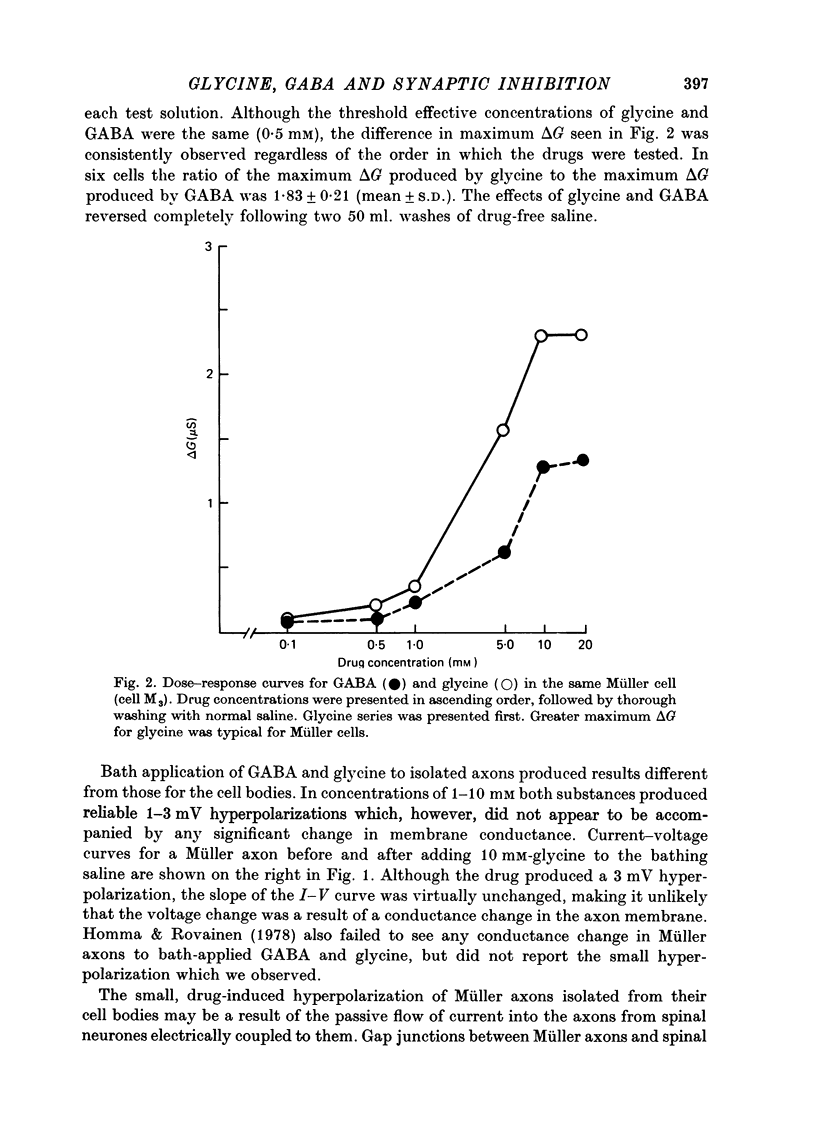
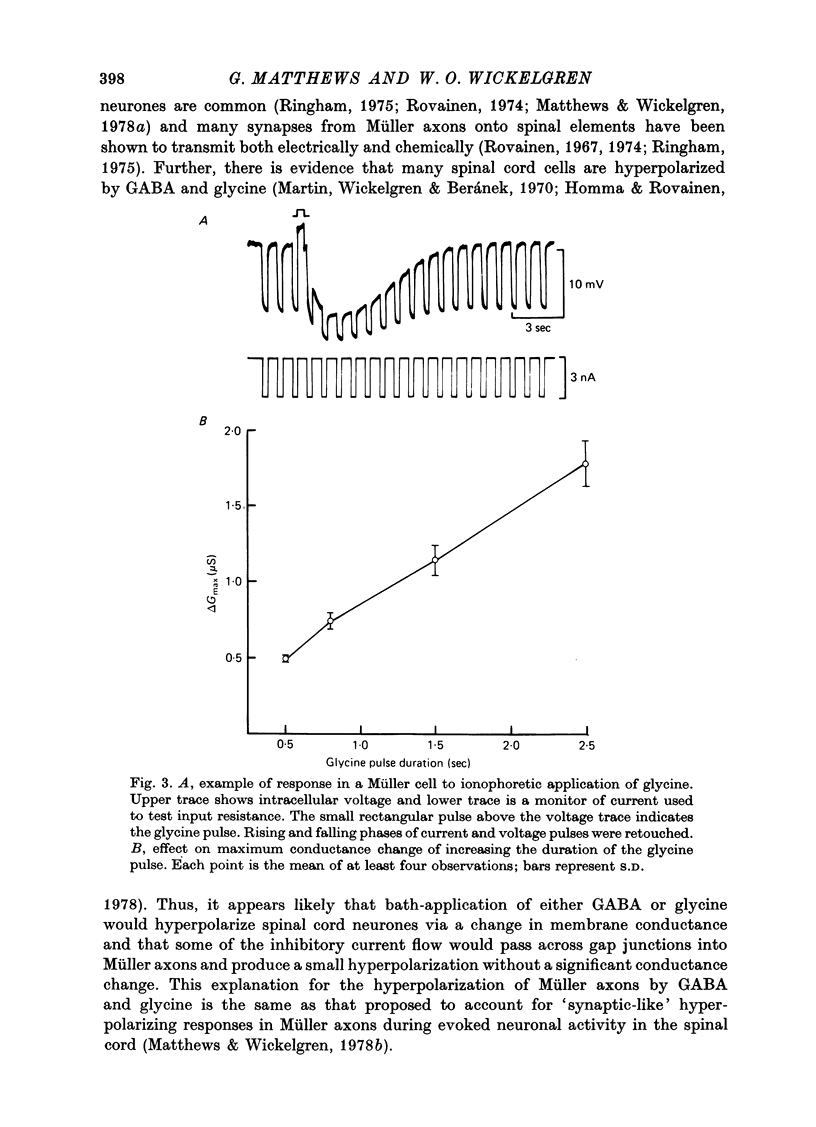
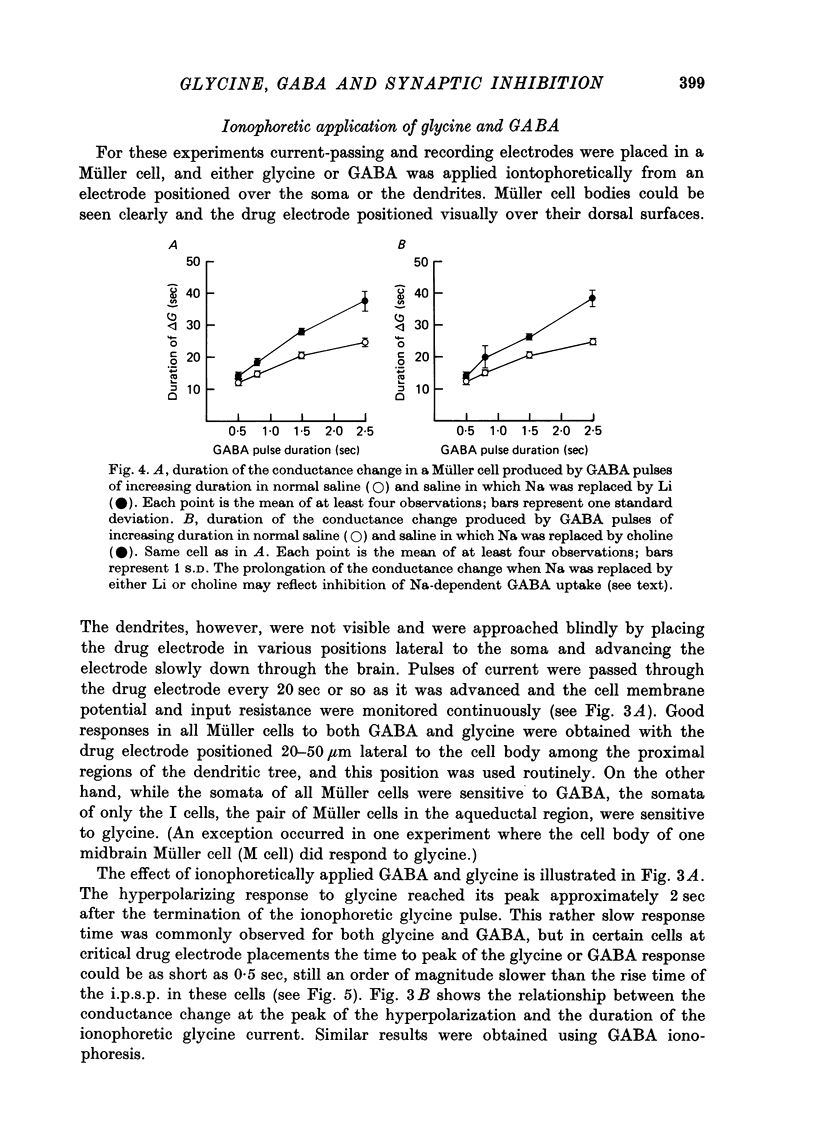
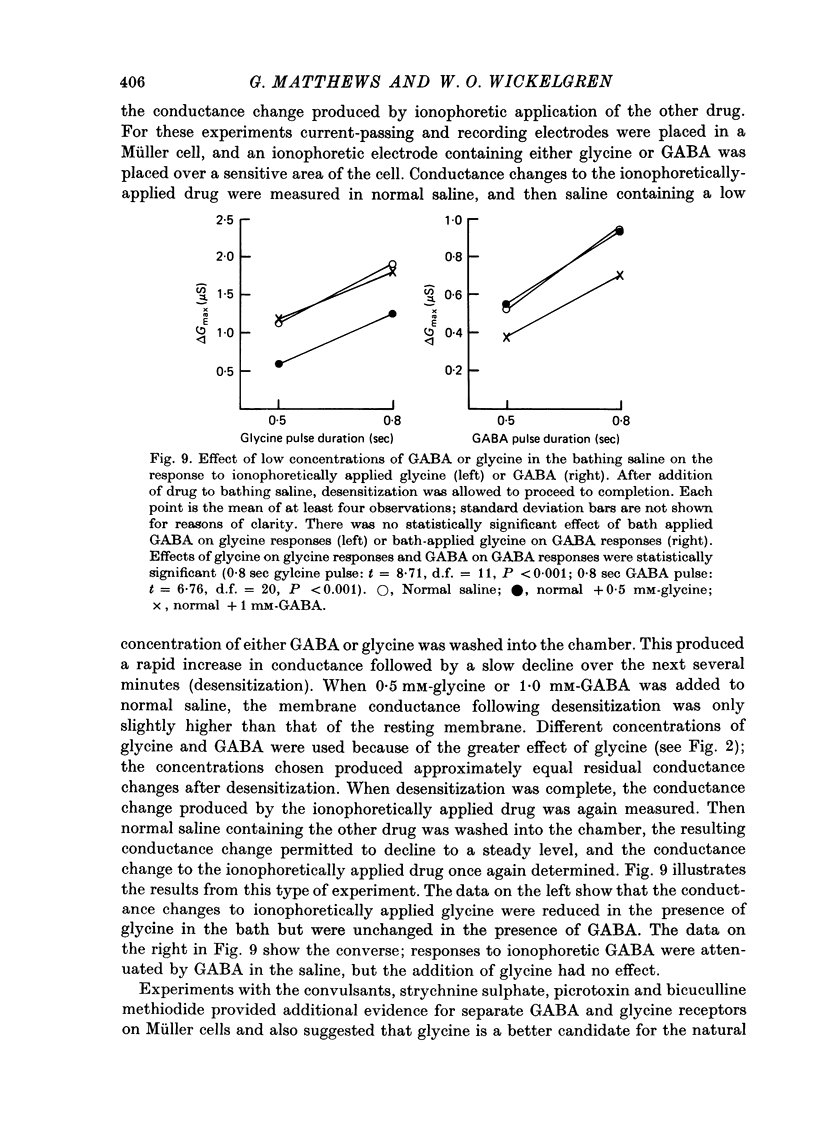
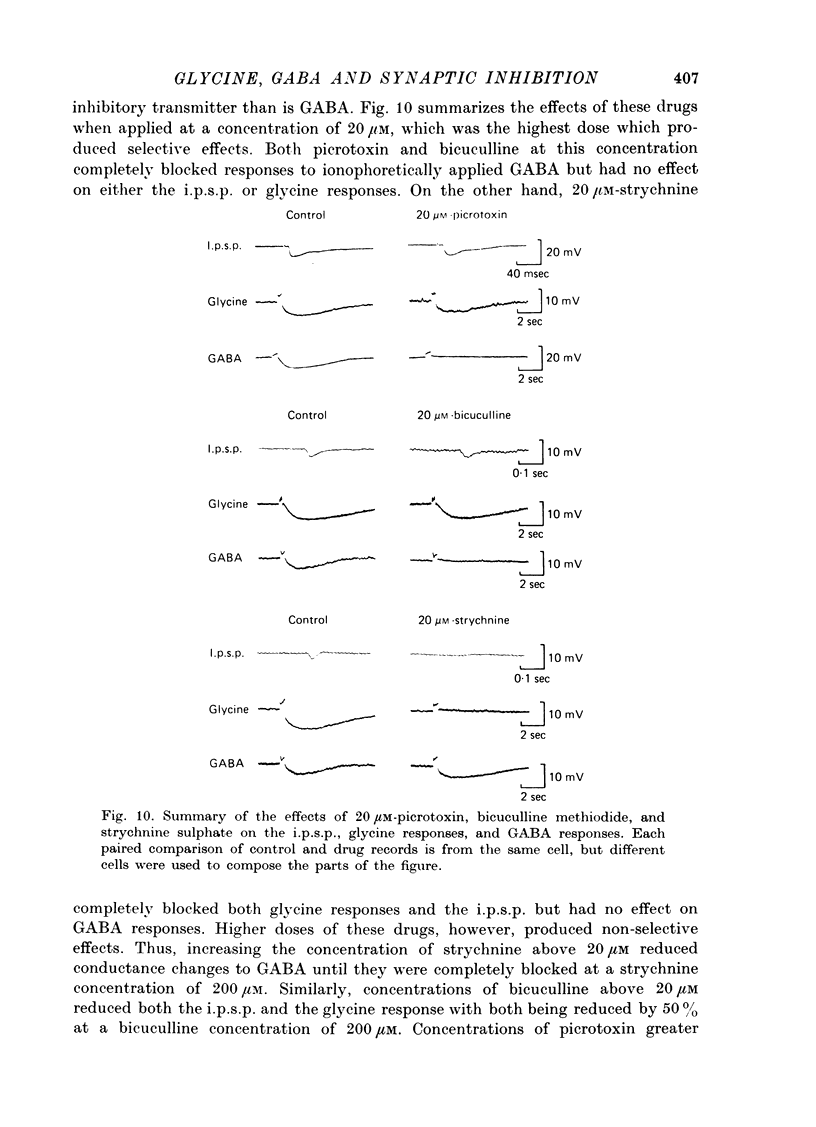
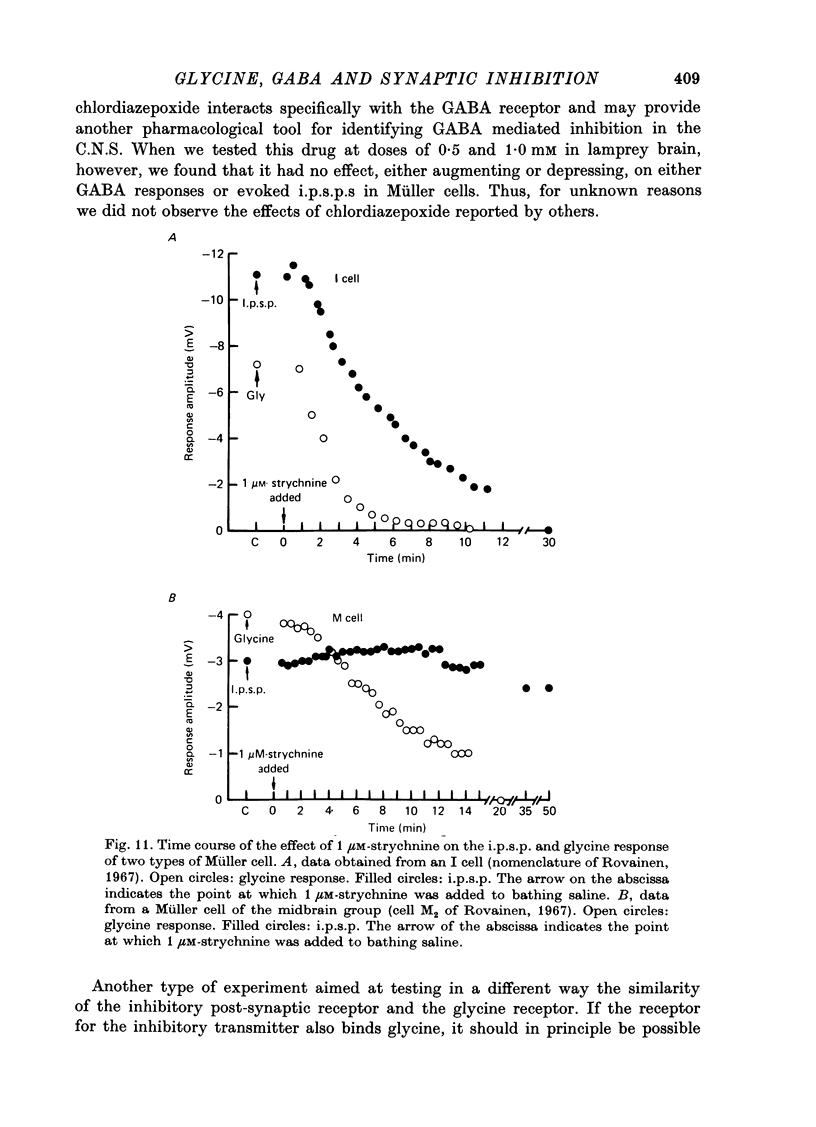
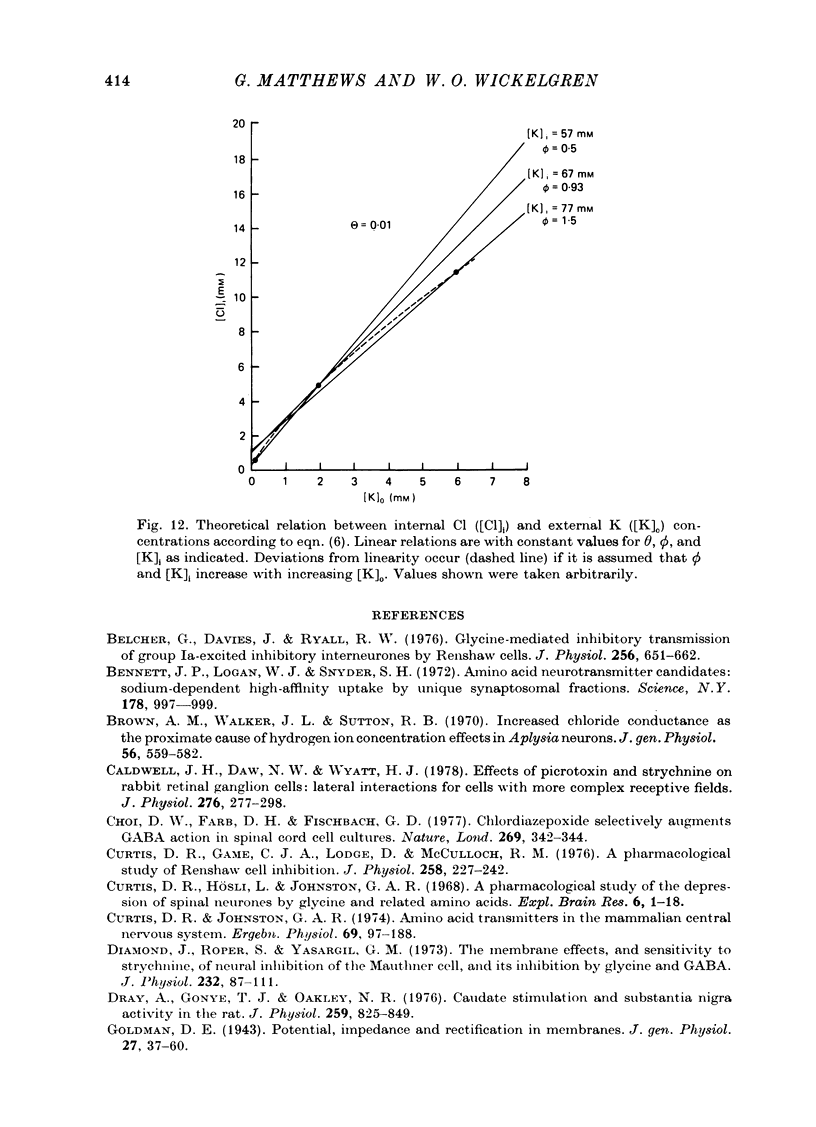
1. Intracellular recordings were made from the cell bodies and axons of giant reticulospinal neurones (Müller cells) of the lamprey and the effects of a variety of putative neurotransmitters tested. Bath-applied acetylcholine, carbamylcholine, norepinephrine, dopamine, histamine and serotonin were without effect. Glycine and gamma-aminobutyric acid (GABA) hyperpolarized and reduced the input resistance of cell bodies but had no effect on the membrane conductance of axons. 2. The threshold dose of bath-applied GABA or glycine for a conductance change in somata was about 0.5 mM and the maximum effect was reached at about 10 mM. The maximum conductance change produced by glycine was always greater than that produced by GABA. 3. Replacement of the sodium in the bathing saline with lithium or choline prolonged the conductance change produced by ionophoretically applied glycine or GABA, suggesting the presence of sodium-dependent uptake systems for glycine and GABA. 4. The reversal potentials for responses to ionophoretically applied glycine and GABA average about --83 mV, the same as that for the inhibitory post-synaptic potential (i.p.s.p.) produced in Müller cells by stimulation of the ipsilateral vestibular nerve. 5. The i.p.s.p. and drug responses appeared to involve an increase in chloride conductance, since their reversal potentials were shifted appropriately by changes in either internal or external chloride. 6. Changes in extracellular potassium concentration also changed i.p.s.p. and drug reversal potentials. However, these effects could be attributed to secondary changes in internal chloride. 7. The receptors for GABA and glycine appeared to be different because of the absence of cross-desensitization and because, at doses below 20 microM, picrotoxin and bicuculline selectively blocked GABA responses while strychnine selectively blocked glycine responses. 8. At concentrations of 20 microM, strychnine eliminated the i.p.s.p. while picrotoxin and bicuculline had no effect. Further, the i.p.s.p. and glycine response of Müller cells located in the isthmic region of the midbrain had the same threshold sensitivity to strychnine. However, the glycine response of other Müller cells was more sensitive to strychnine than was the i.p.s.p. 9. We conclude that glycine is a better candidate for the inhibitory transmitter onto Müller cells than is GABA.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belcher G., Davies J., Ryall R. W. Glycine-mediated inhibitory transmission of group 1A-excited inhibitory interneurones by Renshaw cells. J Physiol. 1976 Apr;256(3):651–662. doi: 10.1113/jphysiol.1976.sp011344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. P., Jr, Logan W. J., Snyder S. H. Amino acid neurotransmitter candidates: sodium-dependent high-affinity uptake by unique synaptosomal fractions. Science. 1972 Dec 1;178(4064):997–999. doi: 10.1126/science.178.4064.997. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Sutton R. B., Walker J. L., Jr Increased chloride conductance as the proximate cause of hydrogen ion concentration effects in Aplysia neurons. J Gen Physiol. 1970 Nov;56(5):559–582. doi: 10.1085/jgp.56.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W., Wyatt H. J. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: lateral interactions for cells with more complex receptive fields. J Physiol. 1978 Mar;276:277–298. doi: 10.1113/jphysiol.1978.sp012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W., Farb D. H., Fischbach G. D. Chlordiazepoxide selectively augments GABA action in spinal cord cell cultures. Nature. 1977 Sep 22;269(5626):342–344. doi: 10.1038/269342a0. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Game C. J., Lodge D., McCulloch R. M. A pharmacological study of Renshaw cell inhibition. J Physiol. 1976 Jun;258(1):227–242. doi: 10.1113/jphysiol.1976.sp011416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Hösli L., Johnston G. A. A pharmacological study of the depression of spinal neurones by glycine and related amino acids. Exp Brain Res. 1968;6(1):1–18. doi: 10.1007/BF00235443. [DOI] [PubMed] [Google Scholar]
- Diamond J., Roper S., Yasargil G. M. The membrane effects, and sensitivity to strychnine, of neural inhibition of the Mauthner cell, and its inhibition by glycine and GABA. J Physiol. 1973 Jul;232(1):87–111. doi: 10.1113/jphysiol.1973.sp010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A., Gonye T. J., Oakley N. R. Caudate stimulation and substantia nigra activity in the rat. J Physiol. 1976 Aug;259(3):825–849. doi: 10.1113/jphysiol.1976.sp011497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma S., Rovainen C. M. Conductance increases produced by glycine and gamma-aminobutyric acid in lamprey interneurones. J Physiol. 1978 Jun;279:231–252. doi: 10.1113/jphysiol.1978.sp012342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Kelly J. S. Uptake and metabolism of gamma-aminobutyric acid by neurones and glial cells. Biochem Pharmacol. 1975 May 1;24(9):933–938. doi: 10.1016/0006-2952(75)90422-0. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J. W. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963 Feb;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. S., Renaud L. P. On the pharmacology of ascending, decending and recurrent postsynaptic inhibition of the cuneo-thalamic relay cells in the cat. Br J Pharmacol. 1973 Jul;48(3):396–408. doi: 10.1111/j.1476-5381.1973.tb08348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLINS L. J., NODA K. THE INFLUENCE OF SODIUM-FREE SOLUTIONS ON THE MEMBRANE POTENTIAL OF FROG MUSCLE FIBERS. J Gen Physiol. 1963 Sep;47:117–132. doi: 10.1085/jgp.47.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R., Barker J. L. Benzodiazepines specifically modulate GABA-mediated postsynaptic inhibition in cultured mammalian neurones. Nature. 1978 Feb 9;271(5645):563–564. doi: 10.1038/271563a0. [DOI] [PubMed] [Google Scholar]
- Martin A. R., Wickelgren W. O., Ber1anek R. Effects of iontophoretically applied drugs on spinal interneurons of the lamprey. J Physiol. 1970 May;207(3):653–665. doi: 10.1113/jphysiol.1970.sp009086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Wickelgren W. O. Evoked depolarizing and hyperpolarizing potentials in reticulospinal axons of lamprey. J Physiol. 1978 Jun;279:551–567. doi: 10.1113/jphysiol.1978.sp012361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Wickelgren W. O. Glutamate and synaptic excitation of reticulospinal neurones of lamprey. J Physiol. 1979 Aug;293:417–433. doi: 10.1113/jphysiol.1979.sp012897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Wickelgren W. O. Sustained depolarizing potentials in reticulospinal axons during evoked seizure activity in lamprey spinal cord. J Neurophysiol. 1978 Mar;41(2):384–393. doi: 10.1152/jn.1978.41.2.384. [DOI] [PubMed] [Google Scholar]
- Meyer H. The different actions of chloride and potassium on postsynaptic inhibition of an isolated neurone. J Exp Biol. 1976 Apr;64(2):477–487. doi: 10.1242/jeb.64.2.477. [DOI] [PubMed] [Google Scholar]
- Motokizawa F., Reuben J. P., Grundfest H. Ionic permeability of the inhibitory postsynaptic membrane of lobster muscle fibers. J Gen Physiol. 1969 Oct;54(4):437–461. doi: 10.1085/jgp.54.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M. J. The uptake of [14C]glycine by slices of mammalian spinal cord. J Physiol. 1971 May;215(1):103–117. doi: 10.1113/jphysiol.1971.sp009460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K., Takeda K., Shinozaki H. Further study on pharmacological properties of the cerebellar-induced inhibition of deiters neurones. Exp Brain Res. 1970 Nov 26;11(4):327–342. doi: 10.1007/BF00237907. [DOI] [PubMed] [Google Scholar]
- Precht W., Yoshida M. Blockage of caudate-evoked inhibition of neurons in the substantia nigra by picrotoxin. Brain Res. 1971 Sep 10;32(1):229–233. doi: 10.1016/0006-8993(71)90171-5. [DOI] [PubMed] [Google Scholar]
- Ringham G. L. Localization and electrical characteristics of a giant synapse in the spinal cord of the lamprey. J Physiol. 1975 Oct;251(2):395–407. doi: 10.1113/jphysiol.1975.sp011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovainen C. M. Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). I. Müller and Mauthner cells. J Neurophysiol. 1967 Sep;30(5):1000–1023. doi: 10.1152/jn.1967.30.5.1000. [DOI] [PubMed] [Google Scholar]
- Rovainen C. M. Synaptic interactions of reticulospinal neurons and nerve cells in the spinal cord of the sea lamprey. J Comp Neurol. 1974 Mar 15;154(2):207–223. doi: 10.1002/cne.901540207. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. The effectiveness of bicuculline as an antagonist of GABA and visually evoked inhibition in the cat's striate cortex. J Physiol. 1975 Sep;250(2):287–304. doi: 10.1113/jphysiol.1975.sp011055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebecis A. K., Di Maria A. Strychnine-sensitive inhibition in the medullary reticular formation: evidence for glycine as an inhibitory transmitter. Brain Res. 1972 May 26;40(2):373–383. doi: 10.1016/0006-8993(72)90140-0. [DOI] [PubMed] [Google Scholar]
- Werman R., Davidoff R. A., Aprison M. H. Inhibitory of glycine on spinal neurons in the cat. J Neurophysiol. 1968 Jan;31(1):81–95. doi: 10.1152/jn.1968.31.1.81. [DOI] [PubMed] [Google Scholar]
- Wickelgren W. O. Physiological and anatomical characteristics of reticulospinalneurones in lamprey. J Physiol. 1977 Aug;270(1):89–114. doi: 10.1113/jphysiol.1977.sp011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward D. J., Hoffer B. J., Siggins G. R., Oliver A. P. Inhibition of Purkinje cells in the frog cerebellum. II. Evidence for GABA as the inhibitory transmitter. Brain Res. 1971 Oct 8;33(1):91–100. doi: 10.1016/0006-8993(71)90308-8. [DOI] [PubMed] [Google Scholar]


