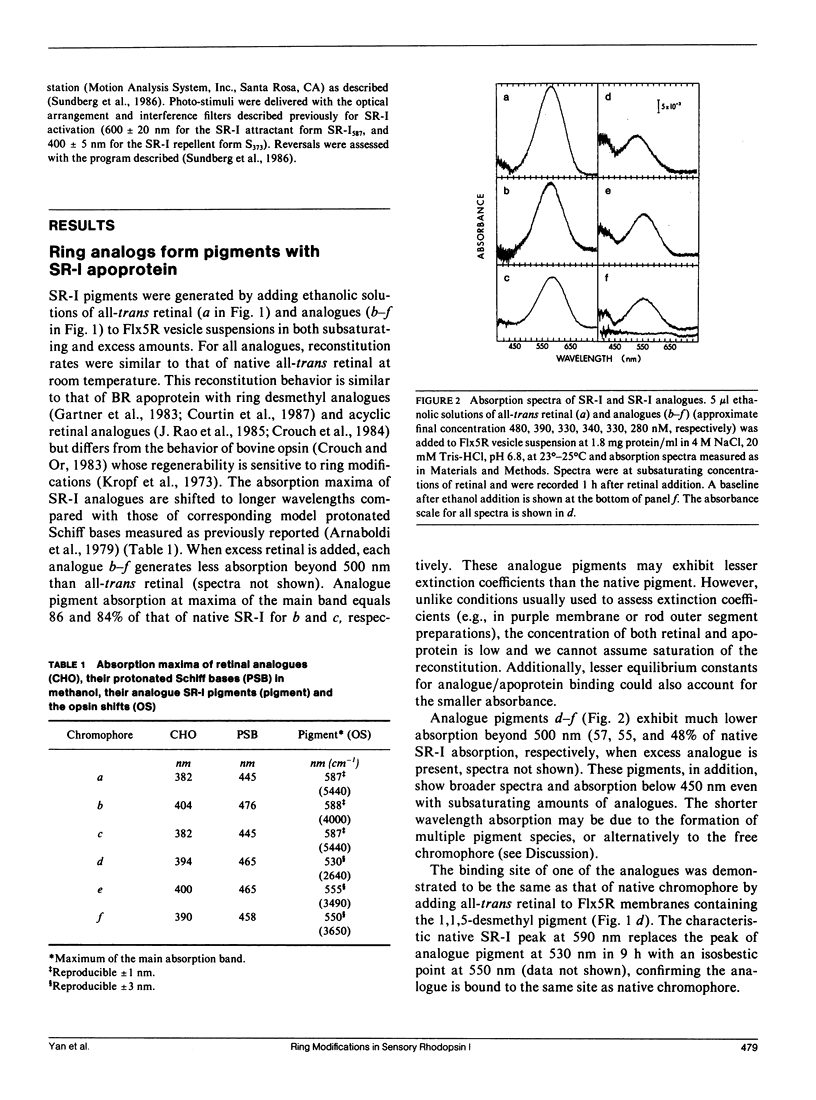
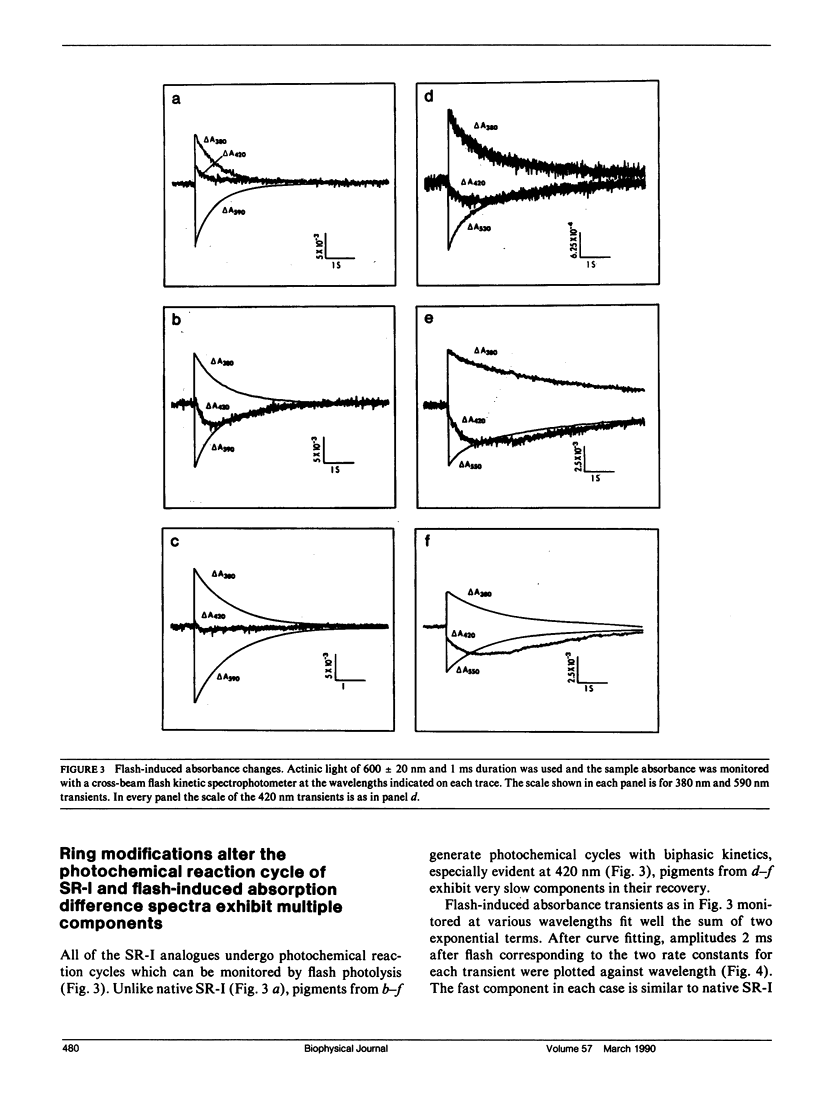
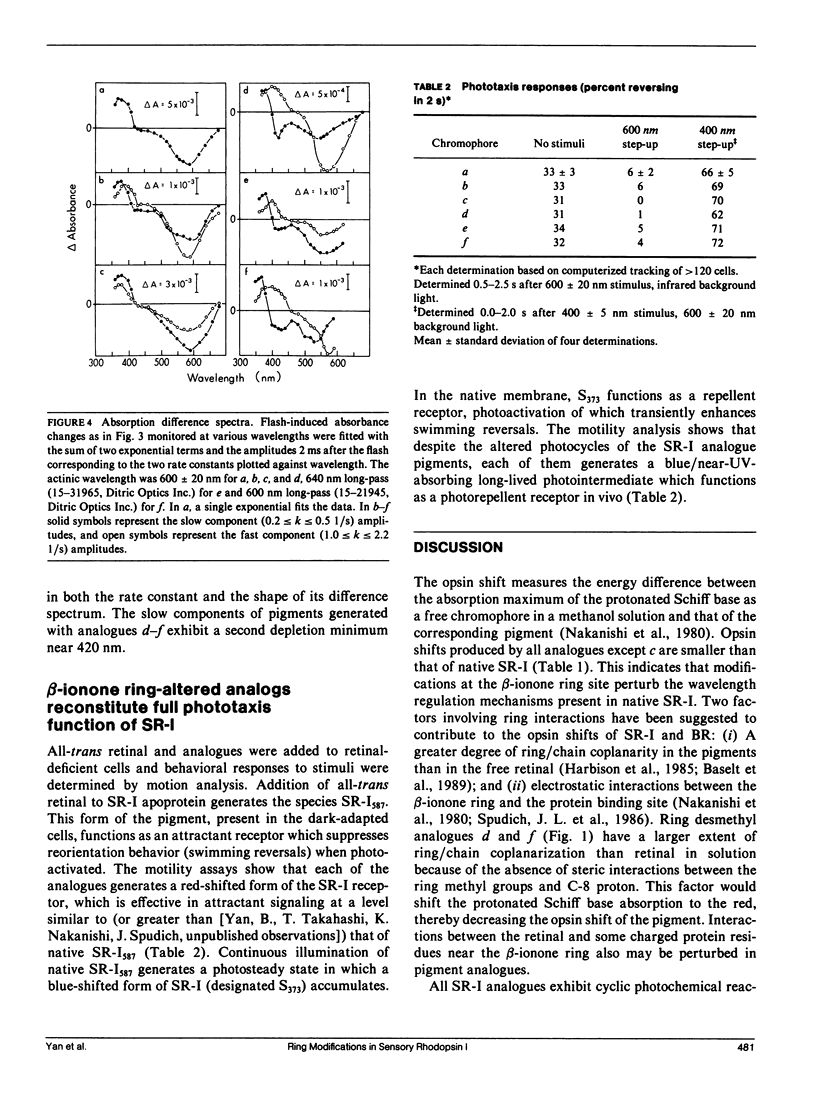
Abstract
Ring desmethyl and acyclic analogues of all-trans retinal were incorporated into the apoprotein of the phototaxis receptor sensory rhodopsin I (SR-I) in Halobacterium halobium membranes. All modified retinals generate SR-I analogue pigments which exhibit "opsin shifts," i.e., their absorption spectra are shifted to longer wavelengths compared with model protonated Schiff bases of the same analogues. Each SR-I pigment analogue exhibits cyclic photochemical reactions as monitored by flash spectroscopy, but the analogue photocycles differ from that of native SR-I by exhibiting pronounced biphasic recovery of flash-induced absorption changes and abnormal flash-induced absorption difference spectra. Despite perturbations in the photochemical properties, the SR-I pigment analogues are capable of both attractant (single photon) and repellent (two photon) phototaxis signaling in cells. Our interpretation is that the hydrophobic ring substituents interact with the binding pocket to maintain the correct configuration for native SR-I absorption and photochemistry, but these interactions are not essential for the physiological function of SR-I as a dual attractant/repellent phototaxis receptor. These results support the conclusion emerging from several studies that the photoactivation process that triggers the conformation changes of SR-I and the related proton pump bacteriorhodopsin is conserved despite the different biological functions of their photoactivation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baselt D. R., Fodor S. P., van der Steen R., Lugtenburg J., Bogomolni R. A., Mathies R. A. Halorhodopsin and sensory rhodopsin contain a C6-C7 s-trans retinal chromophore. Biophys J. 1989 Jan;55(1):193–196. doi: 10.1016/S0006-3495(89)82791-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogomolni R. A., Spudich J. L. The photochemical reactions of bacterial sensory rhodopsin-I. Flash photolysis study in the one microsecond to eight second time window. Biophys J. 1987 Dec;52(6):1071–1075. doi: 10.1016/S0006-3495(87)83301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Govindjee R., Ebrey T., Bagley K. A., Dollinger G., Eisenstein L., Marque J., Roder H., Vittitow J., Fang J. M. Trans/13-cis isomerization is essential for both the photocycle and proton pumping of bacteriorhodopsin. Biophys J. 1985 Apr;47(4):509–512. doi: 10.1016/S0006-3495(85)83944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Courtin J. M., Lugtenburg J., Herzfeld J., Mathies R. A., Griffin R. G. Solid-state 13C NMR detection of a perturbed 6-s-trans chromophore in bacteriorhodopsin. Biochemistry. 1985 Nov 19;24(24):6955–6962. doi: 10.1021/bi00345a031. [DOI] [PubMed] [Google Scholar]
- Hazemoto N., Kamo N., Terayama Y., Kobatake Y., Tsuda M. Photochemistry of two rhodopsinlike pigments in bacteriorhodopsin-free mutant of Halobacterium halobium. Biophys J. 1983 Oct;44(1):59–64. doi: 10.1016/S0006-3495(83)84277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf A., Whittenberger B. P., Goff S. P., Waggoner A. S. The spectral properties of some visual pigment analogs. Exp Eye Res. 1973 Dec 24;17(6):591–606. doi: 10.1016/0014-4835(73)90088-2. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Halorhodopsin: a light-driven chloride ion pump. Annu Rev Biophys Biophys Chem. 1986;15:11–28. doi: 10.1146/annurev.bb.15.060186.000303. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., MacDonald R. E. Light-induced transport in Halobacterium halobium. Methods Enzymol. 1979;56:398–407. doi: 10.1016/0076-6879(79)56038-8. [DOI] [PubMed] [Google Scholar]
- Maeda A., Asato A. E., Liu R. S., Yoshizawa T. Interaction of aromatic retinal analogues with apopurple membranes of Halobacterium halobium. Biochemistry. 1984 May 22;23(11):2507–2513. doi: 10.1021/bi00306a029. [DOI] [PubMed] [Google Scholar]
- Manor D., Hasselbacher C. A., Spudich J. L. Membrane potential modulates photocycling rates of bacterial rhodopsins. Biochemistry. 1988 Aug 9;27(16):5843–5848. doi: 10.1021/bi00416a004. [DOI] [PubMed] [Google Scholar]
- McCain D. A., Amici L. A., Spudich J. L. Kinetically resolved states of the Halobacterium halobium flagellar motor switch and modulation of the switch by sensory rhodopsin I. J Bacteriol. 1987 Oct;169(10):4750–4758. doi: 10.1128/jb.169.10.4750-4758.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. J., Zingoni J. P., Crouch R., Denny M., Liu R. S. Isomers of 3,7,11-trimethyldodeca-2,4,6,8,10-pentaenal (a linear analogue of retinal) and lower homologues in their interaction with bovine opsin and bacterioopsin. Photochem Photobiol. 1985 Feb;41(2):171–174. doi: 10.1111/j.1751-1097.1985.tb03467.x. [DOI] [PubMed] [Google Scholar]
- Spudich E. N., Sundberg S. A., Manor D., Spudich J. L. Properties of a second sensory receptor protein in Halobacterium halobium phototaxis. Proteins. 1986 Nov;1(3):239–246. doi: 10.1002/prot.340010306. [DOI] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Mechanism of colour discrimination by a bacterial sensory rhodopsin. Nature. 1984 Dec 6;312(5994):509–513. doi: 10.1038/312509a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Sensory rhodopsins of halobacteria. Annu Rev Biophys Biophys Chem. 1988;17:193–215. doi: 10.1146/annurev.bb.17.060188.001205. [DOI] [PubMed] [Google Scholar]
- Spudich J. L., McCain D. A., Nakanishi K., Okabe M., Shimizu N., Rodman H., Honig B., Bogomolni R. A. Chromophore/protein interaction in bacterial sensory rhodopsin and bacteriorhodopsin. Biophys J. 1986 Feb;49(2):479–483. doi: 10.1016/S0006-3495(86)83657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Sundberg S. A., Alam M., Spudich J. L. Excitation signal processing times in Halobacterium halobium phototaxis. Biophys J. 1986 Nov;50(5):895–900. doi: 10.1016/S0006-3495(86)83530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka H., Takahashi T., Kamo N., Kobatake Y. Flash spectrophotometric identification of a fourth rhodopsin-like pigment in Halobacterium halobium. Biochem Biophys Res Commun. 1986 Sep 14;139(2):389–395. doi: 10.1016/s0006-291x(86)80003-1. [DOI] [PubMed] [Google Scholar]


