Abstract
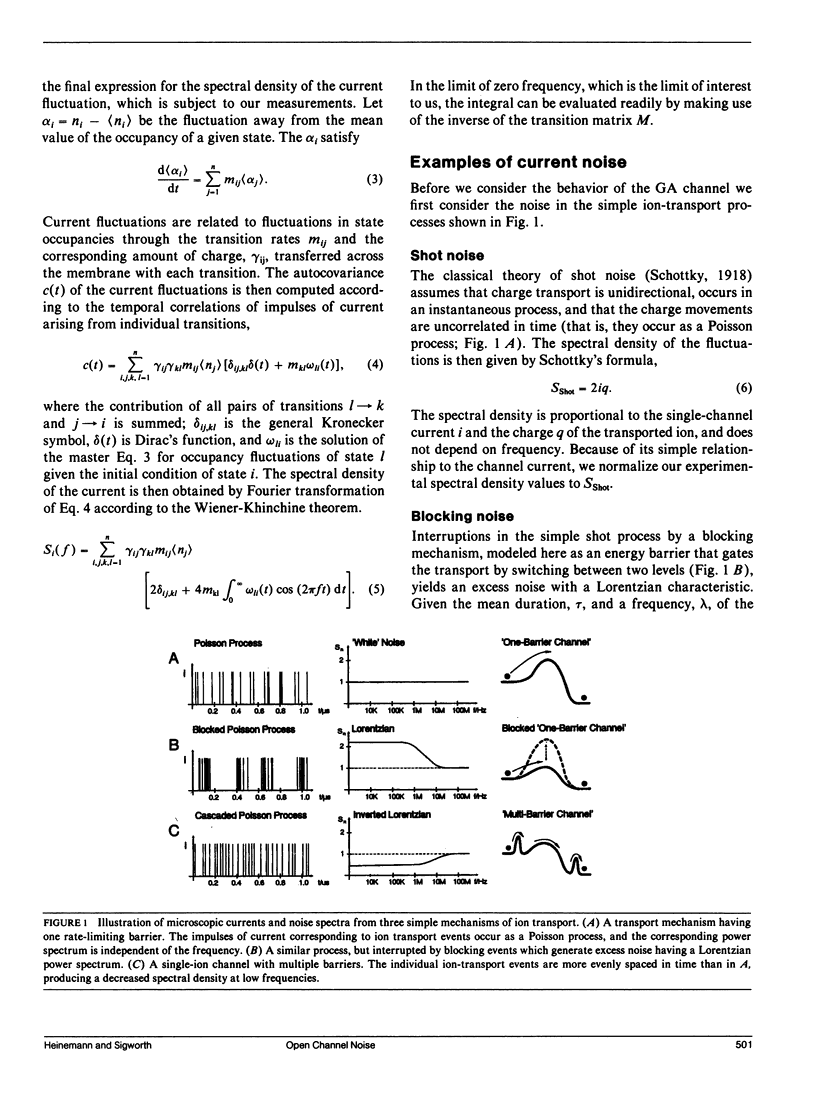
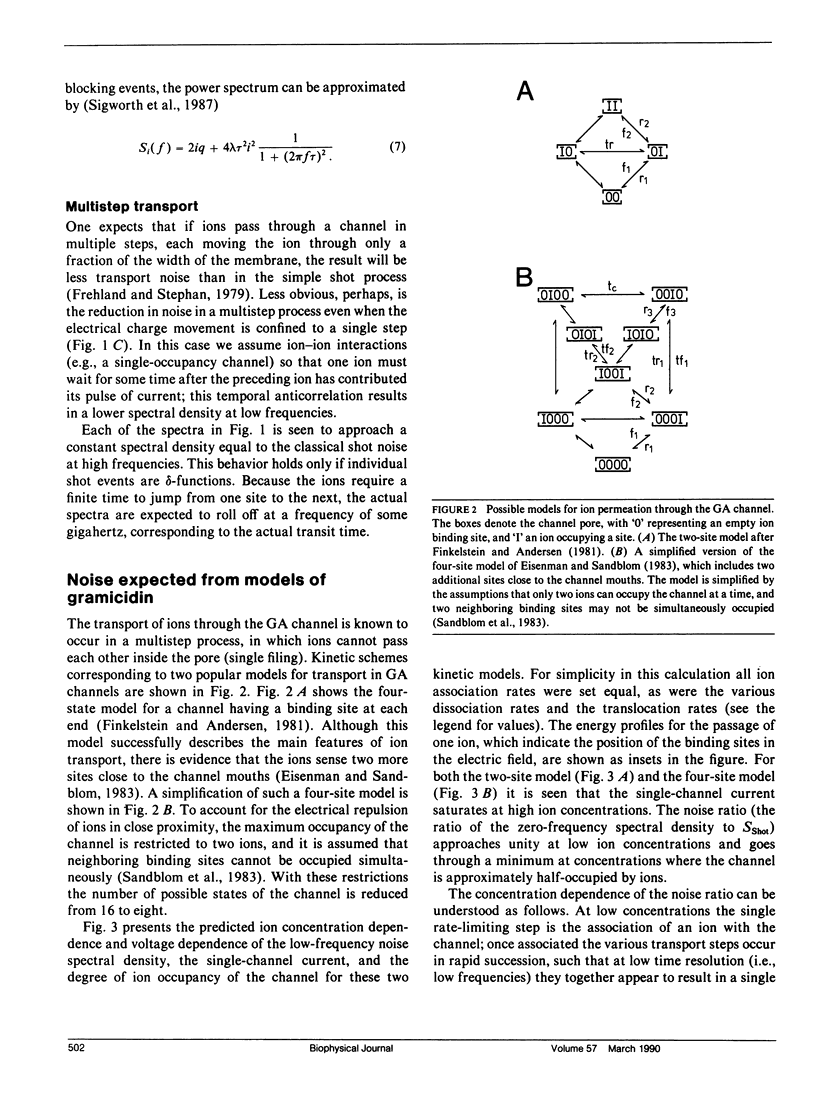
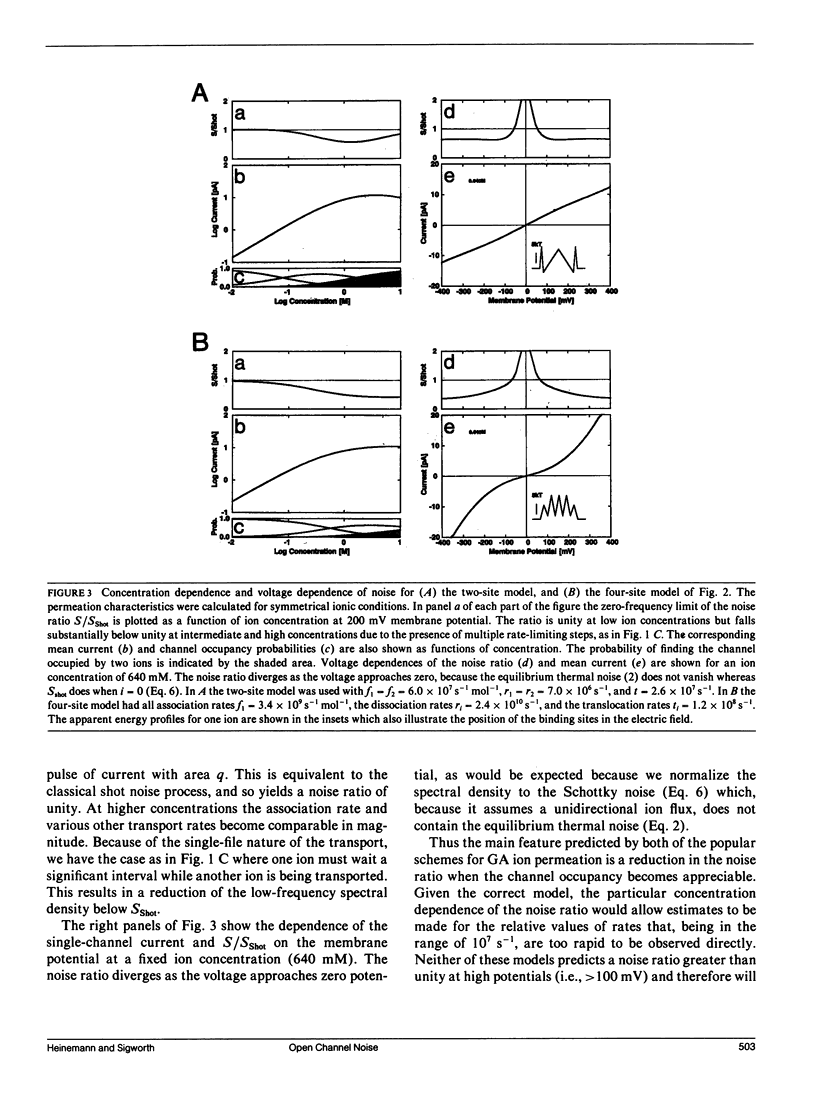
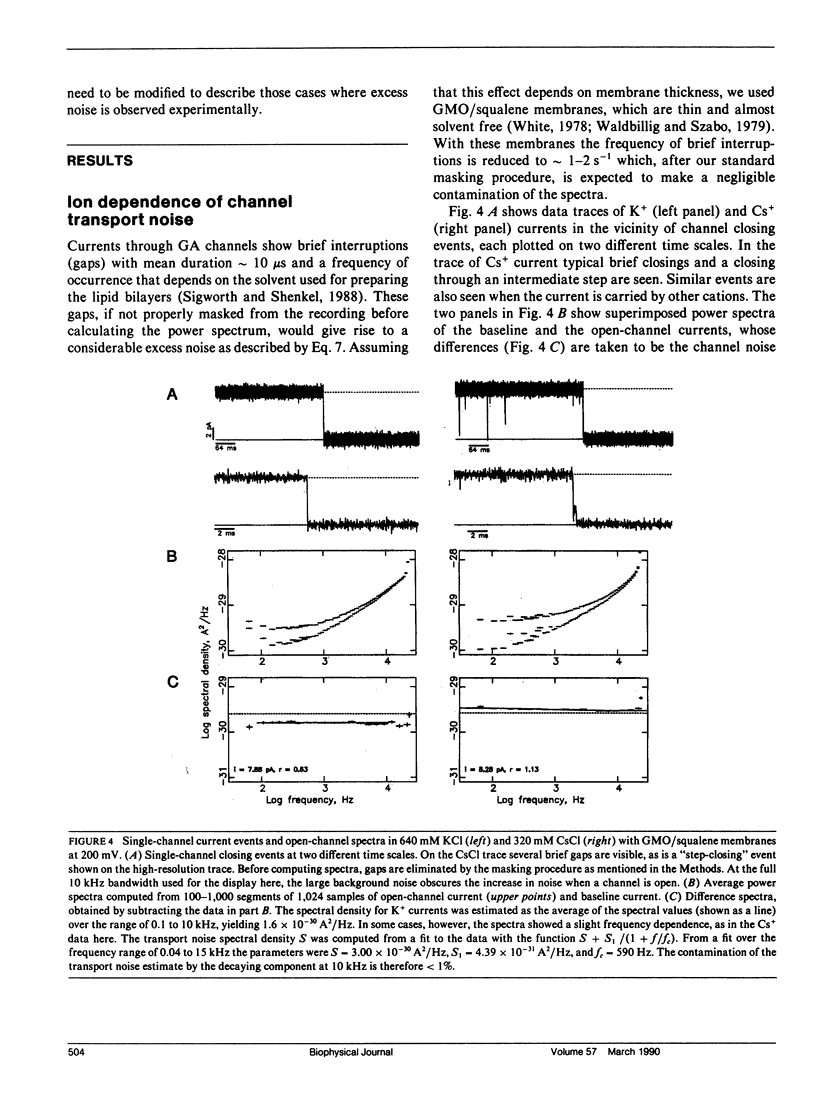
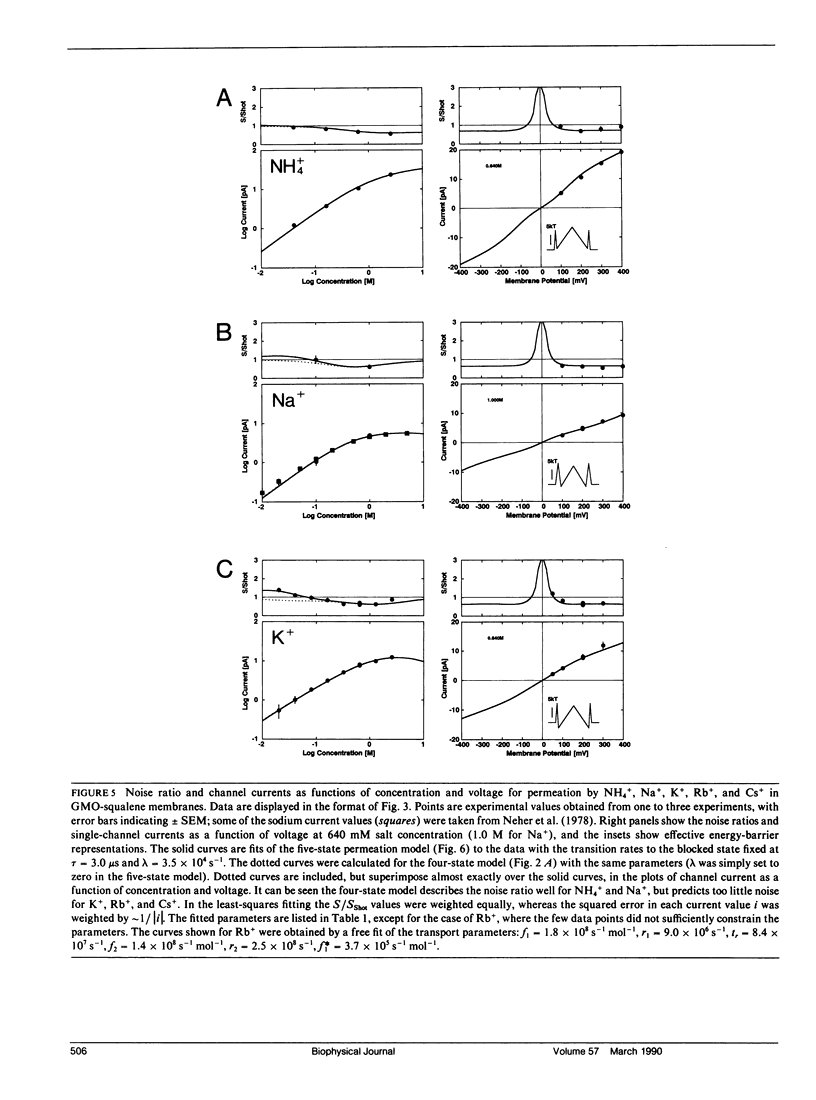
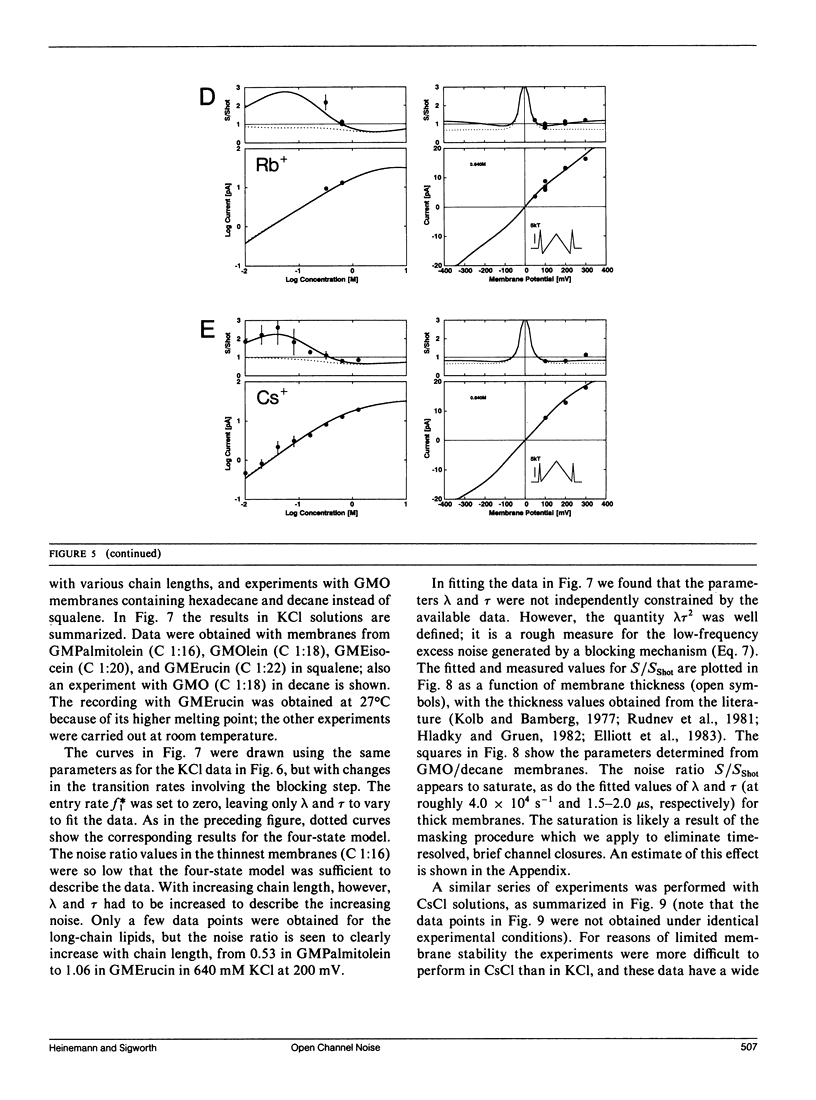
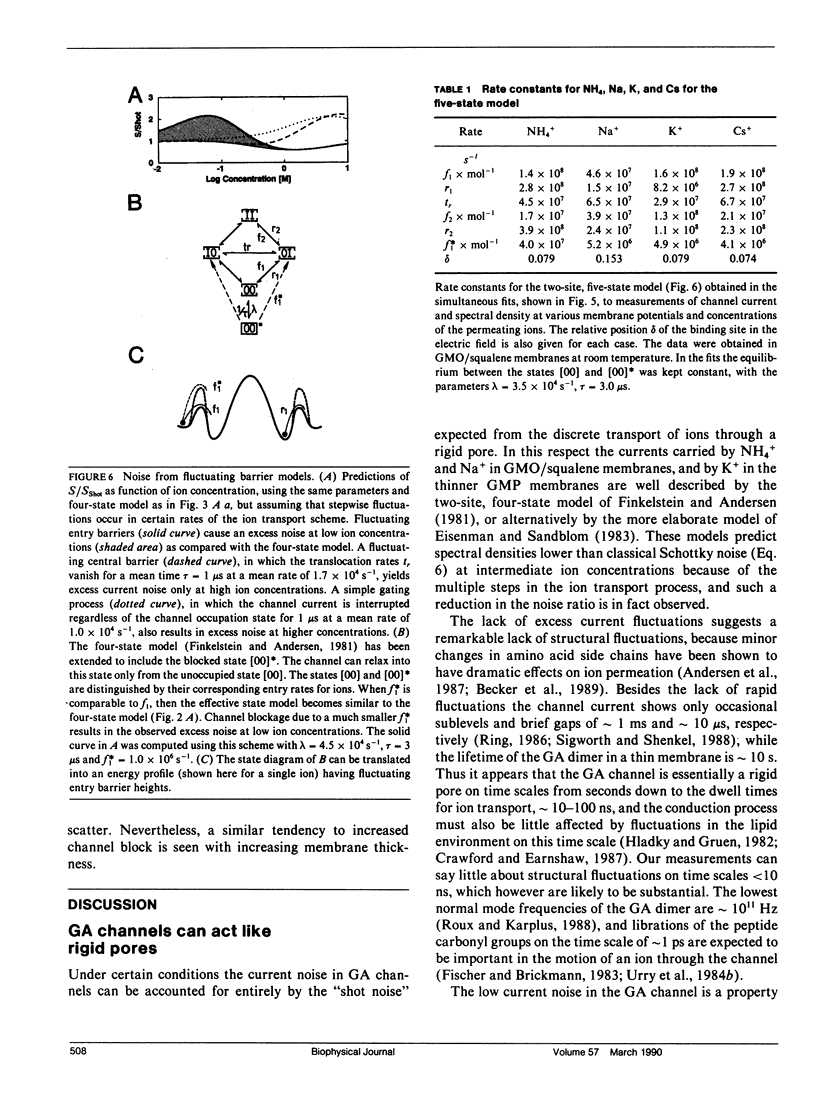
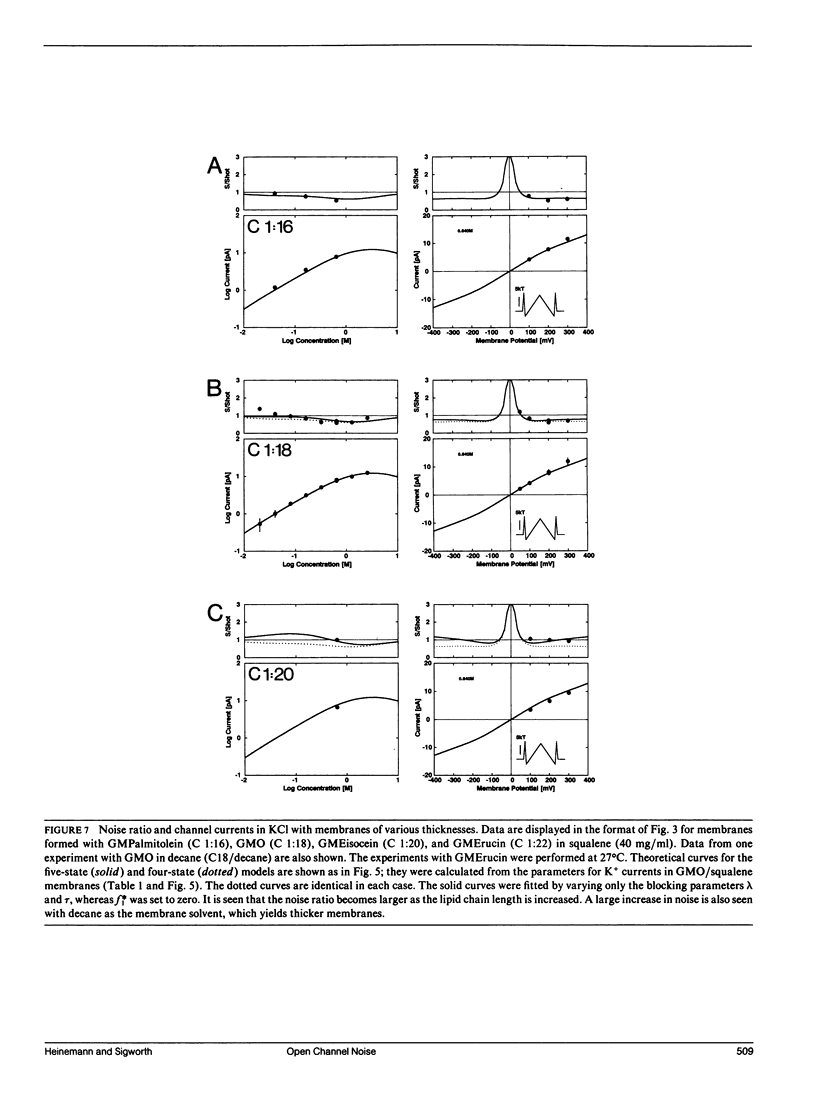
We have measured the fluctuations in the current through gramicidin A (GA) channels in symmetrical solutions of monovalent cations of various concentrations, and compared the spectral density values with those computed using E. Frehland's theory for noise in discrete transport systems (Frehland, E. 1978. Biophys. Chem. 8:255-265). The noise for the transport of NH4+ and Na+ ions in glycerol-monooleate/squalene membranes could be accounted for entirely by "shot noise" in the process of transport through a single-filing pore with two ion binding sites. However, in confirmation of results in a previous paper (Sigworth, F. J., D. W. Urry, and K. U. Prasad. 1987. Biophys. J. 52:1055-1064) currents of Cs+ showed a substantial excess noise at low ion concentrations, as did currents of K+ and Rb+. The excess noise was increased in thicker membranes. The observations are accounted for by a theory that postulates fluctuations of the entry rates of ions into the channel on a time scale of approximately 1 microsecond. These fluctuations occur preferentially when the channel is empty; the presence of bound ions stabilizes the "high conductance" conformation of the channel. The fluctuations are sensed to different degrees by the various ion species, and their kinetics depend on membrane thickness.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arseniev A. S., Barsukov I. L., Bystrov V. F., Lomize A. L., Ovchinnikov YuA 1H-NMR study of gramicidin A transmembrane ion channel. Head-to-head right-handed, single-stranded helices. FEBS Lett. 1985 Jul 8;186(2):168–174. doi: 10.1016/0014-5793(85)80702-x. [DOI] [PubMed] [Google Scholar]
- Auerbach A., Sachs F. Flickering of a nicotinic ion channel to a subconductance state. Biophys J. 1983 Apr;42(1):1–10. doi: 10.1016/S0006-3495(83)84362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford G. E., Earnshaw J. C. Viscoelastic relaxation of bilayer lipid membranes. Frequency-dependent tension and membrane viscosity. Biophys J. 1987 Jul;52(1):87–94. doi: 10.1016/S0006-3495(87)83191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. R., Needham D., Dilger J. P., Haydon D. A. The effects of bilayer thickness and tension on gramicidin single-channel lifetime. Biochim Biophys Acta. 1983 Oct 26;735(1):95–103. doi: 10.1016/0005-2736(83)90264-x. [DOI] [PubMed] [Google Scholar]
- Etchebest C., Pullman A. The gramicidin A channel: energetics and structural characteristics of the progression of a sodium ion in the presence of water. J Biomol Struct Dyn. 1986 Feb;3(4):805–825. doi: 10.1080/07391102.1986.10508463. [DOI] [PubMed] [Google Scholar]
- Finkelstein A., Andersen O. S. The gramicidin A channel: a review of its permeability characteristics with special reference to the single-file aspect of transport. J Membr Biol. 1981 Apr 30;59(3):155–171. doi: 10.1007/BF01875422. [DOI] [PubMed] [Google Scholar]
- Fischer W., Brickmann J. Ion-specific diffusion rates through transmembrane protein channels. A molecular dynamics study. Biophys Chem. 1983 Nov;18(4):323–337. doi: 10.1016/0301-4622(83)80045-3. [DOI] [PubMed] [Google Scholar]
- Frehland E. Current fluctuations in discrete transport systems far from equilibrium. Breakdown of the fluctuation dissipation theorem. Biophys Chem. 1980 Aug;12(1):63–71. doi: 10.1016/0301-4622(80)80040-8. [DOI] [PubMed] [Google Scholar]
- Frehland E. Current noise around steady states in discrete transport systems. Biophys Chem. 1978 Jul;8(3):255–265. doi: 10.1016/0301-4622(78)87007-0. [DOI] [PubMed] [Google Scholar]
- Frehland E., Stephan W. Theory of single-file noise. Biochim Biophys Acta. 1979 May 17;553(2):326–341. doi: 10.1016/0005-2736(79)90236-0. [DOI] [PubMed] [Google Scholar]
- Heinemann S. H., Sigworth F. J. Estimation of Na+ dwell time in the gramicidin A channel. Na+ ions as blockers of H+ currents. Biochim Biophys Acta. 1989 Dec 11;987(1):8–14. doi: 10.1016/0005-2736(89)90448-3. [DOI] [PubMed] [Google Scholar]
- Heinemann S. H., Sigworth F. J. Open channel noise. IV. Estimation of rapid kinetics of formamide block in gramicidin A channels. Biophys J. 1988 Oct;54(4):757–764. doi: 10.1016/S0006-3495(88)83013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladky S. B., Gruen D. W. Thickness fluctuations in black lipid membranes. Biophys J. 1982 Jun;38(3):251–258. doi: 10.1016/S0006-3495(82)84556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. W. Deformation free energy of bilayer membrane and its effect on gramicidin channel lifetime. Biophys J. 1986 Dec;50(6):1061–1070. doi: 10.1016/S0006-3495(86)83550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Vercauteren D. P., Welti M., Chin S., Clementi E. Interaction of K+ ion with the solvated gramicidin A transmembrane channel. Biophys J. 1985 Mar;47(3):327–335. doi: 10.1016/S0006-3495(85)83923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. A., Bamberg E. Influence of membrane thickness and ion concentration on the properties of the gramicidin a channel. Autocorrelation, spectral power density, relaxation and single-channel studies. Biochim Biophys Acta. 1977 Jan 4;464(1):127–141. doi: 10.1016/0005-2736(77)90376-5. [DOI] [PubMed] [Google Scholar]
- Läuger P., Stephan W., Frehland E. Fluctuations of barrier structure in ionic channels. Biochim Biophys Acta. 1980 Oct 16;602(1):167–180. doi: 10.1016/0005-2736(80)90299-0. [DOI] [PubMed] [Google Scholar]
- Läuger P. Transport noise in membranes. Current and voltage fluctuations at equilibrium. Biochim Biophys Acta. 1978 Feb 21;507(2):337–349. doi: 10.1016/0005-2736(78)90427-3. [DOI] [PubMed] [Google Scholar]
- Mackay D. H., Berens P. H., Wilson K. R., Hagler A. T. Structure and dynamics of ion transport through gramicidin A. Biophys J. 1984 Aug;46(2):229–248. doi: 10.1016/S0006-3495(84)84016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sandblom J., Eisenman G. Ionic selectivity, saturation, and block in gramicidin A channels. II. Saturation behavior of single channel conductances and evidence for the existence of multiple binding sites in the channel. J Membr Biol. 1978 Apr 26;40(2):97–116. doi: 10.1007/BF01871143. [DOI] [PubMed] [Google Scholar]
- Ring A. Brief closures of gramicidin A channels in lipid bilayer membranes. Biochim Biophys Acta. 1986 Apr 25;856(3):646–653. doi: 10.1016/0005-2736(86)90160-4. [DOI] [PubMed] [Google Scholar]
- Ring A., Sandblom J. Modulation of gramicidin A open channel lifetime by ion occupancy. Biophys J. 1988 Apr;53(4):549–559. doi: 10.1016/S0006-3495(88)83135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux B., Karplus M. The normal modes of the gramicidin-A dimer channel. Biophys J. 1988 Mar;53(3):297–309. doi: 10.1016/S0006-3495(88)83107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnev V. S., Ermishkin L. N., Fonina L. A., Rovin YuG The dependence of the conductance and lifetime of gramicidin channels on the thickness and tension of lipid bilayers. Biochim Biophys Acta. 1981 Mar 20;642(1):196–202. doi: 10.1016/0005-2736(81)90149-8. [DOI] [PubMed] [Google Scholar]
- Sandblom J., Eisenman G., Hägglund J. Multioccupancy models for single filing ionic channels: theoretical behavior of a four-site channel with three barriers separating the sites. J Membr Biol. 1983;71(1-2):61–78. doi: 10.1007/BF01870675. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. Open channel noise. I. Noise in acetylcholine receptor currents suggests conformational fluctuations. Biophys J. 1985 May;47(5):709–720. doi: 10.1016/S0006-3495(85)83968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. Open channel noise. II. A test for coupling between current fluctuations and conformational transitions in the acetylcholine receptor. Biophys J. 1986 May;49(5):1041–1046. doi: 10.1016/S0006-3495(86)83732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J., Urry D. W., Prasad K. U. Open channel noise. III. High-resolution recordings show rapid current fluctuations in gramicidin A and four chemical analogues. Biophys J. 1987 Dec;52(6):1055–1064. doi: 10.1016/S0006-3495(87)83299-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerra A., Brickmann J. Simulation of voltage-driven hydrated cation transport through narrow transmembrane channels. Biophys J. 1987 Jun;51(6):977–983. doi: 10.1016/S0006-3495(87)83425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Alonso-Romanowski S., Venkatachalam C. M., Bradley R. J., Harris R. D. Temperature dependence of single channel currents and the peptide libration mechanism for ion transport through the gramicidin A transmembrane channel. J Membr Biol. 1984;81(3):205–217. doi: 10.1007/BF01868714. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Alonso-Romanowski S., Venkatachalam C. M., Trapane T. L., Prasad K. U. The source of the dispersity of gramicidin A single-channel conductances. The L X Leu5-gramicidin A analog. Biophys J. 1984 Aug;46(2):259–265. doi: 10.1016/S0006-3495(84)84019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W. The gramicidin A transmembrane channel: a proposed pi(L,D) helix. Proc Natl Acad Sci U S A. 1971 Mar;68(3):672–676. doi: 10.1073/pnas.68.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Trapane T. L., Prasad K. U. Is the gramicidin a transmembrane channel single-stranded or double-stranded helix? A simple unequivocal determination. Science. 1983 Sep 9;221(4615):1064–1067. doi: 10.1126/science.221.4615.1064. [DOI] [PubMed] [Google Scholar]
- Waldbillig R. C., Szabo G. Planar bilayer membranes from pure lipids. Biochim Biophys Acta. 1979 Nov 2;557(2):295–305. doi: 10.1016/0005-2736(79)90328-6. [DOI] [PubMed] [Google Scholar]
- White S. H. Formation of "solvent-free" black lipid bilayer membranes from glyceryl monooleate dispersed in squalene. Biophys J. 1978 Sep;23(3):337–347. doi: 10.1016/S0006-3495(78)85453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


