Abstract
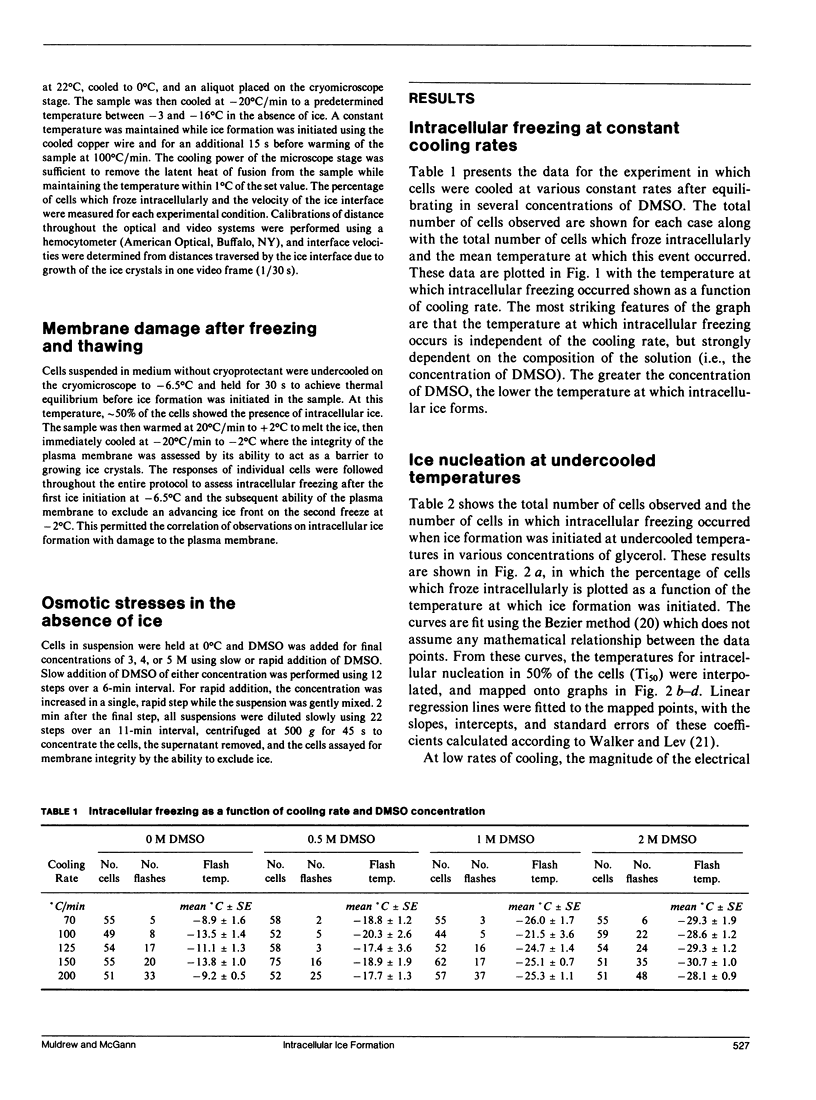
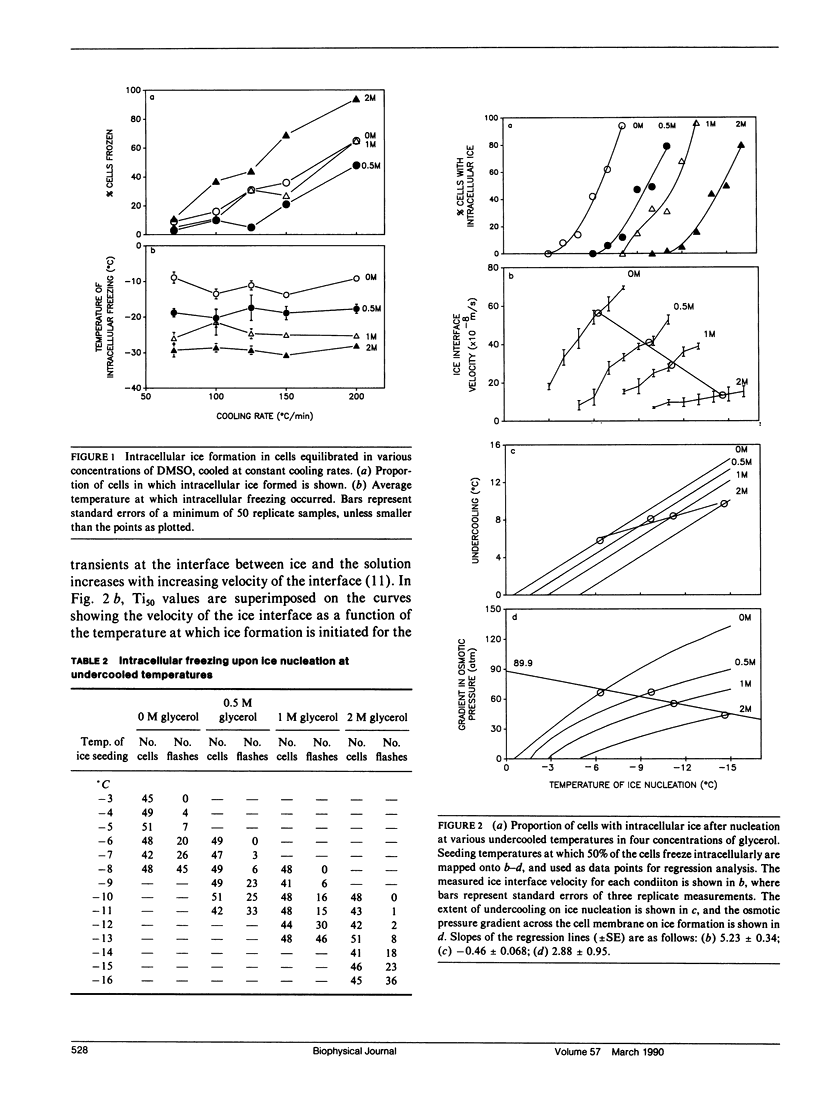
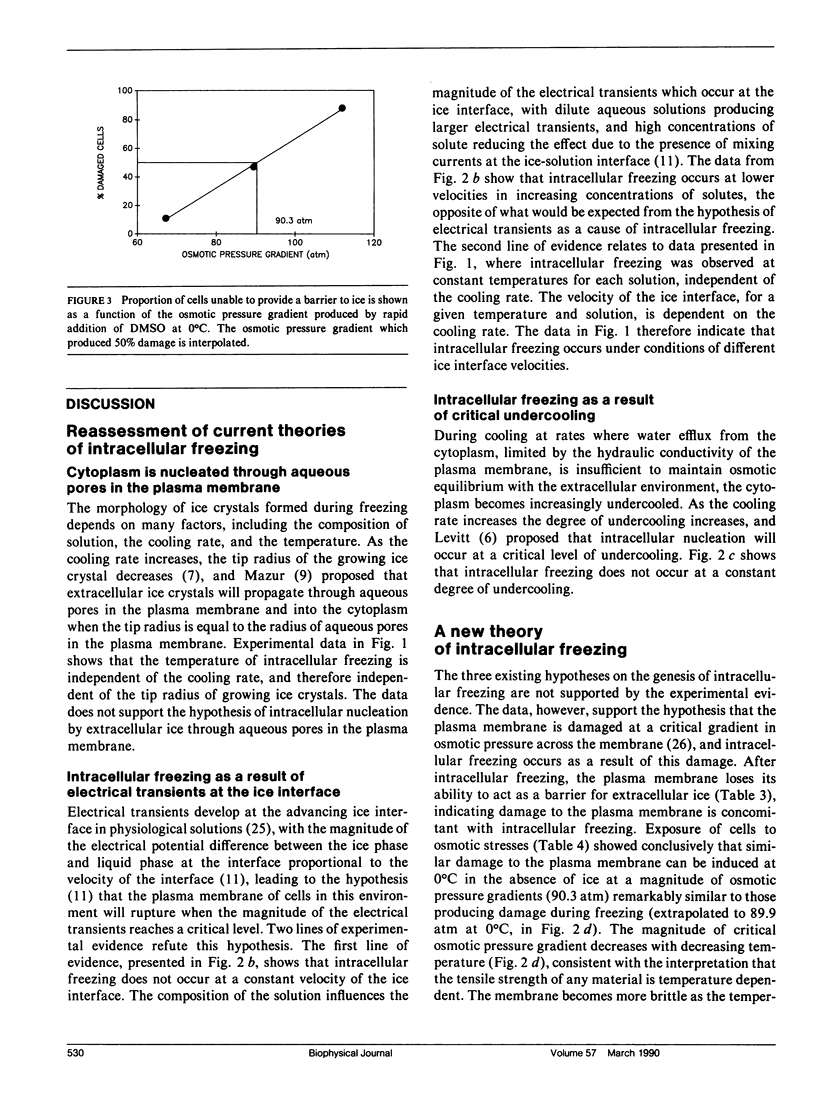
The phenomenon of intracellular freezing in cells was investigated by designing experiments with cultured mouse fibroblasts on a cryomicroscope to critically assess the current hypotheses describing the genesis of intracellular ice: (a) intracellular freezing is a result of critical undercooling; (b) the cytoplasm is nucleated through aqueous pores in the plasma membrane; and (c) intracellular freezing is a result of membrane damage caused by electrical transients at the ice interface. The experimental data did not support any of these theories, but was consistent with the hypothesis that the plasma membrane is damaged at a critical gradient in osmotic pressure across the membrane, and intracellular freezing occurs as a result of this damage. An implication of this hypothesis is that mathematical models can be used to design protocols to avoid damaging gradients in osmotic pressure, allowing new approaches to the preservation of cells, tissues, and organs by rapid cooling.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Diller K. R., Cravalho E. G. A cryomicroscope for the study of freezing and thawing processes in biological cells. Cryobiology. 1970 Nov-Dec;7(4):191–199. doi: 10.1016/0011-2240(70)90021-0. [DOI] [PubMed] [Google Scholar]
- Dowgert M. F., Steponkus P. L. Effect of cold acclimation on intracellular ice formation in isolated protoplasts. Plant Physiol. 1983 Aug;72(4):978–988. doi: 10.1104/pp.72.4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy G. M. Analysis of "solution effects" injury. Equations for calculating phase diagram information for the ternary systems NaCl-dimethylsulfoxide-water and NaCl-glycerol-water. Biophys J. 1980 Nov;32(2):837–850. doi: 10.1016/S0006-3495(80)85019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant J., Morris G. J. Thermal shock and dilution shock as the causes of freezing injury. Cryobiology. 1973 Jun;10(2):134–140. doi: 10.1016/0011-2240(73)90019-9. [DOI] [PubMed] [Google Scholar]
- Farrant J., Walter C. A., Lee H., McGann L. E. Use of two-step cooling procedures to examine factors influencing cell survival following freezing and thawing. Cryobiology. 1977 Jun;14(3):273–286. doi: 10.1016/0011-2240(77)90176-6. [DOI] [PubMed] [Google Scholar]
- Farrant J. Water transport and cell survival in cryobiological procedures. Philos Trans R Soc Lond B Biol Sci. 1977 Mar 29;278(959):191–205. doi: 10.1098/rstb.1977.0037. [DOI] [PubMed] [Google Scholar]
- MAZUR P. KINETICS OF WATER LOSS FROM CELLS AT SUBZERO TEMPERATURES AND THE LIKELIHOOD OF INTRACELLULAR FREEZING. J Gen Physiol. 1963 Nov;47:347–369. doi: 10.1085/jgp.47.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZUR P. Manifestations of injury in yeast cells exposed to subzero temperatures. I. Morphological changes in freeze-substituted and in "frozen-thawed" cells. J Bacteriol. 1961 Nov;82:662–672. doi: 10.1128/jb.82.5.662-672.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZUR P. Physical factors implicated in the death of microorganisms at subzero temperatures. Ann N Y Acad Sci. 1960 Apr 13;85:610–629. doi: 10.1111/j.1749-6632.1960.tb49986.x. [DOI] [PubMed] [Google Scholar]
- Mazur P., Leibo S. P., Chu E. H. A two-factor hypothesis of freezing injury. Evidence from Chinese hamster tissue-culture cells. Exp Cell Res. 1972;71(2):345–355. doi: 10.1016/0014-4827(72)90303-5. [DOI] [PubMed] [Google Scholar]
- Mazur P., Schmidt J. J. Interactions of cooling velocity, temperature, and warming velocity on the survival of frozen and thawed yeast. Cryobiology. 1968 Jul-Aug;5(1):1–17. doi: 10.1016/s0011-2240(68)80138-5. [DOI] [PubMed] [Google Scholar]
- McGann L. E., Farrant J. Survival of tissue culture cells frozen by a two-step procedure to -196 degrees C. II. Warming rate and concentration of dimethyl sulphoxide. Cryobiology. 1976 Jun;13(3):269–273. doi: 10.1016/0011-2240(76)90107-3. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J., Diller K. R. Design and fabrication of a simple, versatile cryomicroscopy stage. Cryobiology. 1982 Oct;L9(5):529–538. doi: 10.1016/0011-2240(82)90182-1. [DOI] [PubMed] [Google Scholar]


