Abstract
Resistance to leishmanial infections depends on intracellular parasite killing by activated host macrophages through the l-arginine-nitric oxide (NO) metabolic pathway. Here we investigate the cell death process induced by NO for the intracellular protozoan Leishmania amazonensis. Exposure of amastigotes to moderate concentrations of NO-donating compounds (acidified sodium nitrite NaNO2 or nitrosylated albumin) or to endogenous NO produced by lipopolysaccharide or gamma interferon treatment of infected macrophages resulted in a dramatic time-dependent cell death. The combined use of several standard DNA status analysis techniques (including electrophoresis ladder banding patterns, YOPRO-1 staining in flow cytofluorometry, and in situ recognition of DNA strand breaks by TUNEL [terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling] assay) revealed a rapid and extensive fragmentation of nuclear DNA in both axenic and intracellular NO-treated amastigotes of L. amazonensis. Despite some similarities to apoptosis, the nuclease activation responsible for characteristic DNA degradation was not under the control of caspase activity as indicated by the lack of involvement of cell-permeable inhibitors of caspases and cysteine proteases. In contrast, exposure of NO-treated amastigotes with specific proteasome inhibitors, such as lactacystin or calpain inhibitor I, markedly reduced the induction of the NO-mediated apoptosis-like process. These data strongly suggest that NO-induced oligonucleosomal DNA fragmentation in Leishmania amastigotes is, at least in part, regulated by noncaspase proteases of the proteasome. The determination of biochemical pathways leading up to cell death might ultimately allow the identification of new therapeutic targets.
Leishmania spp. are obligate intracellular protozoan parasites of macrophages that are responsible for a wide range of human diseases, including self-healing skin lesions, diffuse cutaneous and mucosal lesions, or fatal visceral infections. In the Leishmania life cycle, two principal parasite forms exist: (i) the amastigote form that develops inside mononuclear phagocytes of a vertebrate host and (ii) the motile promastigote form that develops in the vector gut. The various possible outcomes of leishmanial infection have been associated with expansion of specific T helper lymphocyte populations (52). Immune control of leishmaniasis involves a dominant Th1 response, leading to macrophage activation and elimination of intracellular parasites through the induction of nitric oxide synthase (NOS II) and NO synthesis from l-arginine. This prototypical model has been largely evidenced in murine leishmaniasis (17, 22, 33). Human activated macrophages can also induce antileishmanial activity via the l-arginine NO pathway (44, 58, 60). Studies on human cutaneous leishmaniasis reveal that Leishmania killing is associated with NO production (40). Moreover, the capacity of canine macrophages to eliminate intracellular amastigotes through a NO-dependent mechanism has been documented (46, 55, 59).
NO-dependent cytostatic and/or cytotoxic activities by activated macrophages on various parasites have been clearly demonstrated (20, 26, 29, 33, 57). In Leishmania infections, NO-generating creams applied topically to lesions revealed a modest efficacy in mouse models, whereas they were able to cure human patients (15, 65). Moreover, studies have shown that successful chemotherapy in a murine and canine model of visceral leishmaniasis depended on upregulation of the l-arginine NO pathway (14, 58). However, the cellular and molecular mechanisms whereby NO exerts its cytotoxic activity are not yet well understood. NO toxicity results from complex phenomena involving several molecular targets. In antitumoral models, impairment of essential cellular functions, including mitochondrial respiration, DNA synthesis by blocking ribonucleotide reductase (31), and enzyme inactivation through Fe/S center alterations or S nitrosylation, have been documented (16, 19). Recent studies found that several parasite targets may be affected by NO toxicity in Leishmania parasites. These targets include metabolic enzymes, such as GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and aconitase (29, 32) or cysteine proteinase (51).
Apoptosis represents an important mechanism by which exogenous or endogenous NO mediates toxicity in mammalian cells (1, 36, 41, 42, 63). We investigated the genomic DNA status of NO-treated Leishmania amazonensis amastigotes. Several apoptosis detection methods (appearance of the characteristic DNA ladder banding pattern on agarose gels and in situ TUNEL [terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling] and flow cytofluorometry assays) revealed that Leishmania cell death was associated with extensive nuclear DNA fragmentation. Cell-permeable caspase inhibitors did not modify NO-mediated nuclear DNA fragmentation. In contrast, endonuclease activation could be a consequence of Ca2+-sensitive calpain and/or proteasome activity. These data strongly suggest that NO can induce damage in the unicellular pathogen Leishmania by a coordinated process of intracellular protein degradation mediated by the proteasome, which may lead to nuclear DNA fragmentation. An apoptosis-like cell death pathway could represent an important and highly regulated mechanism used for the clearance of Leishmania within infected macrophages stimulated to produce NO endogenously or during treatments with NO-releasing drugs.
MATERIALS AND METHODS
Reagents.
Fetal calf serum (FCS) was obtained from Dutscher S.A. RPMI 1640 medium (lot 0MB0174) was purchased from BioWhittaker Europe, and l-glutamine (lot 3414) was from BioMedia. Penicillin-streptomycin (10,000 IU/ml to 10,000 UG/ml; lot 3037222) and phosphate buffer saline (Ca2+ and Mg2+ free) were obtained from Life Technologies. Bacterial lipopolysaccharide (LPS), gamma interferon (IFN-γ; mouse recombinant), bovine serum albumin (BSA; fraction V, lot 59H0696), and MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; lot 66H5033) were purchased from Sigma. RNase was obtained from Eurogentec, proteinase K was from Promega, and agarose was purchased from Eurobio. YOPRO-1 iodide was provided by Molecular Probes. Nitro-l-arginine, inhibitory peptides Z-DEVD-CMK and Z-VAD-FMK, E-64d [(2S,3S)-trans-epoxysuccinyl-l-leucylamido-3-methylbutane ethyl ester], calpain inhibitor I, lactacystin, proteasome inhibitor II, and MG-115 were purchased from Alexis Biochemicals. All other cellular-grade and molecular biology-grade chemicals were obtained from Sigma. Cell-free MAA/20 culture medium for axenically grown amastigotes was defined according to the method of Lemesre et al. (29, 30). Nitrosylated albumin was prepared as previously described (38).
Animals.
Female BALB/c mice (Iffa Credo, Saint-Germain-sur-l'Arbresle, France) were housed under conventional conditions and given water and chow ad libitum. The use of the animals conformed to institutional guidelines.
Parasites and in vitro cultures.
A cloned line of L. amazonensis (MHOM/BR/76/LTB-012) was used in all experiments. L. amazonensis axenically grown amastigote forms were maintained at 32 ± 1°C by weekly subpassages in MAA/20 medium. From a starting inoculum of 5 × 105 amastigote forms/ml, a cell density of ca. 2 × 107 parasites/ml was obtained on day 7. Axenically grown amastigote forms appeared homogeneous, round to ovoid, without apparent flagella, and nonmotile. Axenically grown amastigotes from various Leishmania species clearly resembled intracellular amastigotes with regard to their ultrastructural, biological, biochemical, and immunological properties (5, 6, 13, 24, 29, 30, 53). Cell concentrations were determined by daily counts with a hemocytometer at a ×400 magnification after adequate dilution in phosphate-buffered saline (PBS) containing 0.25% glutaraldehyde.
Treatment with the NO donors sodium nitrite (NaNO2) and nitrosylated albumin (NO-BSA).
Late-log-phase amastigote forms were washed three times in PBS-0.01 M (pH 4.5) before treatment. Parasites (107/ml) were resuspended in the same buffer containing 5 mM sodium nitrite. After 20 min, 2 h, 4 h, 6 h, and 12 h of incubation at 32 ± 1°C in the dark, respectively, parasites were washed in Ca2+-Mg2+-free PBS (0.01 M, pH 7.2) before analysis. Control amastigotes were treated in the same way but in the absence of sodium nitrite.
Albumin or nitrosylated albumin (4 mg/ml) was added to the parasite cultures. After incubation for 20 min, 4 h, or 12 h at 32 ± 1°C, the amastigotes were washed three times in Ca2+-Mg2+-free PBS (0.01 M, pH 7.2) before analysis.
Assessment of NO donors' effect on amastigote survival.
NO-treated and untreated amastigotes (2 × 108/ml) were resuspended in MAA/20 medium for each incubation time. The MTT-based microassay was used to estimate NO-induced death as described before (53). Tetrazolium bromide has the property of being reduced by parasitic dehydrogenases into a dark blue insoluble formazan product, the amount of which depends on the number of viable amastigotes. Briefly, parasites (100 μl) were seeded in a 96-well microplate. After a 1-h incubation, 10 μl of MTT (10 mg/ml) was added to each well, and the plates were further incubated for 4 h. The enzyme reaction was then stopped by the addition of 100 μl of 50% isopropanol-10% sodium dodecyl sulfate. The plates were incubated for an additional 30 min while being agitated at room temperature before the optical density value at 570 nm was determined with a Titer-Tech 96-well scanner (Labsystems Multiscan EX).
In vitro macrophage infections.
Mouse peritoneal macrophages were washed with prewarmed RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 2 mM glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml and cultured overnight in 16-well Lab-Tek tissue culture slides (Nalge Nunc International). Nonadherent cells were removed by two washes with prewarmed RPMI medium, and then the macrophages were infected with stationary-phase extracellular amastigotes at a parasite/macrophage ratio of 3:1 for 2 h at 34°C with 5% CO2. Noninternalized parasites were removed by gentle washing. Infected macrophages were then cultured in the presence or absence of the following reagents: LPS (10 ng/ml), gamma interferon (IFN-γ; 100 U/ml), and a combination of LPS and IFN-γ. Controls were made by adding 1 mM nitro-l-arginine, a competitive inhibitor of NOS II, or 1 mM nitro-l-arginine plus 2 mM l-arginine to revert the inhibition.
Culture supernatants were collected for nitrite production measurement 48 h later. Macrophages were then washed with prewarmed RPMI, fixed with methanol, and stained with Giemsa for parasite counts or processed for the TUNEL technique (see below). The percent parasite index (PI) inhibition compared to the untreated controls was calculated as follows: percent PI = 100 − [(mean number of amastigotes per macrophage × percent infected macrophages in treated wells)/(mean number of amastigotes per macrophage × percentage of infected macrophages in untreated wells)] × 100. The results were taken as the mean of duplicate experiments.
Measurement of nitrite production.
NO2− accumulation in the medium over 48 h for the leishmanicidal assay was used as an indicator of NO production and was assayed by the Griess reaction (50). Briefly, 60 μl of Griess reagent A (1% sulfanilamide in 1.2 N HCl) and 60 μl of Griess reagent B [0.3% N-(1-naphthyl)ethylenediamine] were added to 100 μl of each supernatant in triplicate wells in a 96-well plate. Plates were read at 540 nm in an enzyme-linked immunosorbent assay plate reader (Labsystems Multiskan EX). NaNO2 in RPMI was used to construct a standard curve for each plate reading.
In situ TUNEL assay.
Slides with infected macrophages or axenic amastigotes were fixed for 20 min with PBS containing 4% paraformaldehyde, washed twice with 0.01 M PBS (pH 7.2), and stored at −20°C until use. DNA fragmentation was analyzed in situ by using a colorimetric apoptosis detection system (Promega, Madison, Wis.) according to the manufacturer's instructions. After performance of the TUNEL protocol, preparations were analyzed under a microscope at ×1,000 magnification. Apoptotic nuclei appeared dark brown. Other nuclei were not colored.
DNA agarose gel electrophoresis.
Cell pellets were incubated in lysis buffer (10 mM Tris, 10 mM EDTA, 0.5% Triton X-100 [pH 7.4]) for 30 min at 4°C. Qualitative analysis of DNA fragmentation was performed as previously described (7) by agarose gel electrophoresis of DNA extracted from 5 × 108 axenically grown amastigotes. DNA was then visualized under UV light after the gels were stained with ethidium bromide.
Flow cytofluorometry analysis with YOPRO-1.
The percentage of apoptotic cells was quantitated by flow cytofluorometric analysis by using the DNA intercalatant YOPRO-1 as previously described (25). Briefly, 106 L. amazonensis amastigotes were incubated with 10 μM YOPRO-1 for 10 min. Cells were immediately analyzed with a FACScan flow cytometer (Becton Dickinson, Ivry, France) by using an argon-ion laser tuned to 488 nm. Green cell fluorescence, gated on forward scatter and side light scatter, was collected by using a (525 ± 10)-nm band-pass filter (FL1) and displayed by using a logarithmic amplification.
Statistical analysis.
Statistical significance was analyzed by Student's t test. All experiments were performed at least twice.
RESULTS
Kinetics of NO-mediated effect on L. amazonensis amastigote viability.
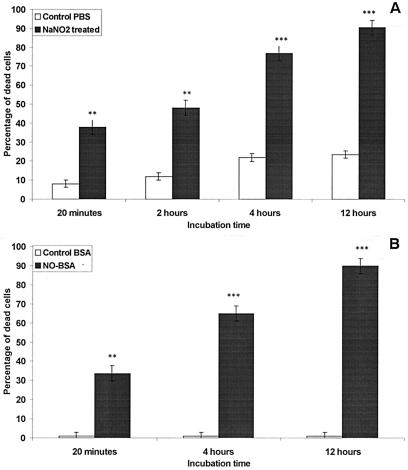
Treatment of L. amazonensis extracellular amastigotes with both NO donors led to a dramatic loss of parasite viability with regard to morphological changes such as decreased cell volume under microscopic examination (data not shown), as well as a decrease in dehydrogenase activities as determined by the MTT micromethod (Fig. 1). Exposure of axenically grown amastigotes to NO generated from acidified 5 mM NaNO2 resulted in a time-dependent cell death (Fig. 1A). Significant toxicity could be detected as early as 20 min after treatment with the chemical (ca. 40% of amastigote mortality). Survival gradually decreased; <10% of the microorganisms were viable after a 12-h exposure to NO generated from NaNO2. Incubation of amastigotes in PBS (pH 4.5) in the absence of added NaNO2 did not significantly affect parasite viability in the first few hours, and fewer than 25% amastigotes were dead after 12 h (Fig. 1A).
FIG. 1.
Effects of 5 mM acidified sodium nitrite (A) or 4 mg of nitrosylated albumin/ml (B) on the death of L. amazonensis amastigotes. Parasite viability was assessed by the MTT micromethod as described by Sereno and Lemesre (53). Statistical significance was determined by the Student's t test. Results are expressed as the mean and standard deviation of three independent experiments made in triplicate. Statistical significance: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
About 30% of amastigotes exposed to 4 mg of NO-BSA/ml were killed within the first 20 min of incubation, reaching ca. 65 and 90% after 4 and 12 h of incubation, respectively (Fig. 1B). The addition of albumin alone at 4 mg/ml failed to affect amastigote viability, even after 12 h of incubation (Fig. 1B), and no striking morphological change was observed under the microscope (not shown).
The relevance of the observations above would remain questionable unless similar effects could be observed in intracellular amastigotes of L. amazonensis exposed to endogenous NO produced by activated macrophages. Mouse peritoneal macrophages infected with axenically cultured amastigotes and activated for 24 and 48 h with IFN-γ and LPS led to a time-dependent intracellular killing of Leishmania as determined by the reduction of PIs of ca. 55% at 24 h and 90% at 48 h (Fig. 2B), concomitant with NO2− accumulation in culture fluids (Fig. 2A). NO2− production and antileishmanial activity both decreased in the presence of 1 mM nitro-l-arginine, a competitive inhibitor of NOS II. They were almost totally restored by the addition of 2 mM l-arginine (Fig. 2). Inhibition of the PI did not exceed 20% when incubation conditions did not induce NO production (Fig. 2B).
FIG. 2.
NO-dependent cytotoxicity against L. amazonensis in activated mouse macrophages. Macrophages were infected with L. amazonensis amastigotes at a cell/parasite ratio of 1:3 in RPMI 1640 medium supplemented with 10% FCS. Infected cells were treated with LPS, IFN-γ, LPS plus IFN-γ, LPS plus IFN-γ plus nitro-l-arginine (a competitive inhibitor of NOS II), and LPS plus+ IFN-γ plus nitro-l-arginine plus l-arginine (to reverse NOS II inhibition). NO2− levels in supernatants (A) and PI inhibitions in cells (B) were determined 24 and 48 h later. The results represent the mean and standard deviation of two independent experiments made in duplicate. Statistical significance: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
NO-mediated apoptosis-like DNA fragmentation of L. amazonensis amastigotes.
NO-mediated DNA fragmentation exhibiting features of apoptosis was first assessed by using extracellular amastigotes incubated 5 mM NaNO2 and monitoring the genomic DNA status of treated versus untreated parasites. As shown in Fig. 3 (lanes 5, 7, and 9), nuclear DNA fragmentation into oligonucleosomal-sized fragments (720, 360, and 180 bp), a typical feature of apoptotic cells, was readily visible in the case of NO-treated amastigotes. Untreated amastigotes did not show any DNA fragmentation, even after 6 h of incubation in acidic conditions (Fig. 3, lanes 2, 4, 6, and 8). Interestingly, NO-mediated nuclear DNA fragmentation (720-bp multimer) could be detected as early as after 2 h of contact with the NO donor (Fig. 3, lane 5). After 6 h of contact with the chemical, the entire genomic DNA of amastigotes was mostly fragmented into oligonucleosome-sized DNA of 360 and 180 bp (Fig. 3, lane 9), the end products of DNA alteration in apoptosis.
FIG. 3.
DNA fragmentation analysis. Agarose gel electrophoresis of untreated amastigote (suspended in PBS [pH 4.5], lanes 2, 4, 6, and 8) and NO-treated amastigote (suspended in PBS [pH 4.5] plus 5 mM NaNO2; lanes 3, 5, 7, and 9) DNA (5 μg) is shown. Exposure times: 20 min (lanes 2 and 3), 2 h (lanes 4 and 5), 4 h (lanes 6 and 7), and 6 h (lanes 8 and 9); molecular weights are indicated beside lanes 1 and 10. The experiment was done four times.
Endonuclease activity was also evaluated by the TUNEL assay. As shown in Fig. 4C, nuclei of axenically grown amastigotes exposed to 5 mM NaNO2 were intensely stained dark brown after a 4-h incubation in contrast to those of untreated parasites (Fig. 4A). Corresponding controls incubated without terminal deoxynucleotidyl transferase (TdT) enzyme in order to determinate the specificity of the reaction did not show any staining (Fig. 4B and D). Interestingly, kinetoplast DNA of NO-treated and untreated amastigotes was also stained. Indeed, replication of kinetoplast DNA involves the release of catenated minicircles from the network with subsequent DNA 3′-OH tail production stained by biotinylated dUTP via the TdT enzyme. Similarly, the in situ TUNEL technique revealed that the nuclear DNA fragmentation of intracellular amastigotes also occurred in Leishmania-infected stimulated macrophages (Fig. 5). Moreover, a strong correlation between nuclear DNA fragmentation, increased NO2− levels, and antileishmanial activity was observed (Fig. 2 and 5). Labeled amastigote nuclei could only be visualized inside activated macrophages producing NO (Fig. 5D and F). Nuclei of intracellular amastigotes, which we can visualized after Giemsa staining, were free of label in nonactivated macrophages (Fig. 5A) and in macrophages stimulated with LPS alone (B), with IFN-γ alone (C), or with LPS and IFN-γ plus 1 mM nitro-l-arginine (E). In the absence of TdT enzyme, corresponding negative controls did not exhibit any staining, thus demonstrating the specificity of the reaction (Fig. 5G and H).
FIG. 4.
In situ analysis of L. amazonensis extracellular amastigote death exhibiting features of apoptosis. (A and B) Untreated (PBS [pH 4.5], 4 h) amastigotes (A) and, as negative control, untreated parasites incubated without TdT (B). (C and D) NO-treated amastigotes (5 mM NaNO2, 4 h) incubated with (C) or without (D) TdT. DNA fragmentation analysis was determined by the TUNEL method and analyzed under a microscope at ×1,600 magnification. The experiment was done twice.
FIG. 5.
In situ analysis of the apoptosis-like process of L. amazonensis amastigotes in mouse macrophages. Cells were infected with amastigote forms at a parasite/cell ratio of 3:1 in RPMI 1640 medium supplemented with 10% FCS. Infected cells were activated for 48 h. (A) Untreated macrophages; (B) macrophages activated with LPS; (C) macrophages activated with IFN-γ; (D) macrophages activated with LPS plus IFN-γ; (E) macrophages activated with LPS plus IFN-γ plus nitro-l-arginine (a competitive inhibitor of NO synthase type II); (F) macrophages activated with LPS plus IFN-γ plus nitro-l-arginine plus l-arginine (to reverse inhibition of NOS-II). (G and H) Negative controls, either activated macrophages (G) or nonactivated macrophages (H), were incubated without TdT. DNA fragmentation analysis was determined by the TUNEL technique and analyzed under a microscope at ×1,000 magnification (A to H). A magnification of ×1,600 was used to identify labeled amastigotes in panel I. The experiment was done twice.
Quantitation and time course of NO-mediated cell death of L. amazonensis amastigotes.
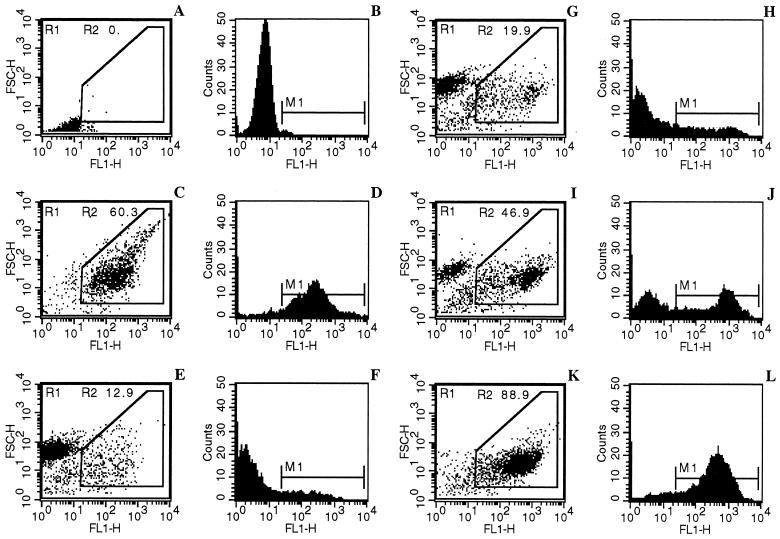
The impermeable DNA intercalated YOPRO-1 has been previously used for monitoring the apoptosis of Trypanosoma cruzi epimastigotes (3, 25). We show here that it could also selectively differentiate viable, necrotic (sodium dodecyl sulfate-treated), and apoptotic amastigotes (Fig. 6). Trivalent antimonial (potassium antimonyl tartrate at 50 μg/ml) was used as a positive apoptosis inducer as described by Sereno et al. (54). Antimonial-mediated apoptotic cells (Fig. 6C and D) were easily distinguished from living (Fig. 6E and F) and necrotic cells (Fig. 6A and B) by using the combined analysis of their different patterns of FSC-H properties and YOPRO-1 staining (FL1-H).
FIG. 6.
Analysis of the NO-induced apoptosis-like process in extracellular amastigotes by a cytofluorometry YOPRO-1 differential staining technique. Parasites were incubated for 6 h in 0.01 M PBS (pH 7.2) (negative control, not shown), treated for 10 min by 0.1% sodium dodecyl sulfate (necrosis control, A and B), and treated for 24 h with 50 μg of potassium antimonyl tartrate/ml (apoptosis control, C and D). Amastigotes were, respectively, incubated in 0.01 M PBS (pH 4.5) as an internal control (a 6-h incubation is depicted in E and F) and treated with 5 mM NaNO2 for 2 h (G and H), 4 h (I and J), or 6 h (K and L). The apoptotic cell percentage (R2), corresponding to both reduced forward scatter and high fluorescence intensity, is indicated in each experimental condition. M1 shows the peak of fluorescence intensity in panels B, D, F, H, J, and L. The experiment was done three times in duplicate.
The time course of the apoptosis-like changes was determined by evaluating the percentage of cells that exhibited the maximum fluorescence seen in NO-treated amastigotes. Amastigotes incubated in 0.01 M PBS (pH 4.5) for 20 min, 2 h, and 4 h (not shown) and 6 h (Fig. 6E and F) displayed a homogeneous population of living cells with a low fluorescence background. In contrast, a new cell population with a high FL1 fluorescence corresponding to apoptotic cell-like cells appeared in NO-treated amastigotes. This population increased as a function of exposure time to the NO donor, representing ca. 14% at 20 min (not shown), 19.9% at 2 h (Fig. 6G and H), 46.9% at 4 h (Fig. 6I and J), and 88.9% at 6 h (Fig. 6K and L). Interestingly, the time courses of YOPRO-1 staining and of viability loss were quite similar (Fig. 1 and 6). Altogether, these data indicated that NO directly and quickly induced extracellular amastigote death through a process that mimics apoptosis.
Effects of apoptosis inhibitors.
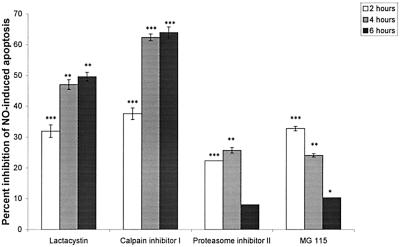
In higher eukaryote cells, apoptosis often occurs downstream of the death signal by protease activation, leading to endonuclease ignition (21). In these instances, cysteine proteases of the caspase family, Ca2+-sensitive calpains, or proteasomes can initiate cell death. We used here cytofluorometric analysis with YOPRO-1 staining to test the relevance of these pathways with apoptosis inhibitors. Amastigote treatment with apoptosis-blocking agents did not alter the viability of untreated amastigotes during the time course experiments (not shown). Whereas caspase inhibitors reversed the apoptosis of murine peritoneal macrophages induced by 4 mM butyrate as recently described (47), inhibitors of caspase 3 (inhibitory peptide Z-DEVD-CMK at 5 μM), caspase 1 (inhibitory peptide Z-VAD-FMK at 5 μM), or cysteine proteases (E-64d at 20 μM] had no effect on the cell death induced by NO (data not shown). In contrast, a significant time-dependent inhibitory effect was observed on the NO-mediated apoptosis-like phenomenon with specific irreversible proteasome inhibitors (10 μM lactacystin and 20 μM calpain inhibitor I) (Fig. 7). Treatment with reversible proteasome inhibitors (10 μM proteasome inhibitor II or 10 μM MG-115) exhibited a less potent and an inversely time-dependent inhibitory activity. In the first 2 h of treatment, all of the proteasome inhibitors used exerted an inhibitory effect ranging from 20 to 40%. After a 4-h incubation with proteasome inhibitor II and MG-115, NO-induced DNA changes were reduced by ca. 25%, whereas lactacystin and calpain inhibitor I produced marked inhibitions of 47 and 62%, respectively. These percentages reached ca. 50 and 64%, respectively, after 6 h of incubation (Fig. 7). All of these data support the view that the proteasome pathway could be involved in promoting apoptosis-like changes in NO-exposed amastigotes.
FIG. 7.
Percentage of apoptotic cells, in the presence of proteasome inhibitors, as measured by flow cytometry and YOPRO-1 staining of NO-treated amastigotes of L. amazonensis (5 mM NaNO2). Incubation media were supplemented with the following proteasome inhibitors: lactacystin (10 μM), calpain inhibitor I (20 μM), proteasome inhibitor II (10 μM), and MG-115 (10 μM). Statistical significance was determined by the Student's t test. The results are the means ± the standard deviation of three independent experiments made in duplicate. Statistical significance: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
DISCUSSION
In this study we showed that the leishmanicidal effect of NO seemed to be the consequence of the induction of a programmed cell death-like process. NO donors induced a pattern of DNA fragmentation typical of an apoptotic process in axenic amastigotes of L. amazonensis. This apoptotic phenomenon was confirmed by both the TUNEL technique and flow cytometry. This typical aspect of apoptosis was also demonstrated in the Leishmania intracellular parasite stage inside NO-producing macrophages, by using the combination IFN-γ and LPS, which is mainly used as a potent inducer of NOS II.
NO also seems to be able to induce necrosis, as well as apoptosis (34). Interestingly, NO-mediated apoptosis has been extensively studied in mammalian cells (1, 2, 12, 18, 28, 36, 63). Recent reports have shown that unicellular organisms may kill themselves by an evolutionarily conserved programmed cell death pathway (i.e., apoptosis) (4). This pathway has been described in several lower organisms, including the slime mold Dictyostelium discoideum (11) and several parasitic protozoa. The effect of chloroquine on a sensitive strain of the malaria parasite P. falciparum led to an oligonucleosomal DNA fragmentation, suggesting that apoptosis may be involved in the action of chloroquine on the parasite (45). Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense procyclic forms, upon treatment with the lectin concanavalin A, died by a process similar to apoptosis in mammals (61, 62); changes in cytoskeletal organization, cytosol vacuolization, and nuclear DNA fragmentation were reported. Apoptosis-like changes have also been reported for T. cruzi in response to conditioned medium or antibiotic G418 (3). A shift in the distribution of EF-1α to a nuclear localization was reported as one of the changes associated with cell death (8). In leishmaniasis, a heat shock was able to induce in promastigotes of L. amazonensis a DNA cleavage into an oligonucleosomal ladder characteristic of cells dying by apoptosis (39). In the same way, luteolin and quercetin, two plant-derived flavonoids, inhibited cell cycle progression in L. donovani promastigotes, leading to apoptosis (37). Recently, we demonstrated that the antileishmanial toxicity of trivalent antimonials is associated with parasite oligonucleosomal DNA fragmentation, a finding indicative of the occurrence of late events in the overall apoptosis process (54). However, the activation of an apoptosis-like machinery in the parasite stage directly in contact with macrophage antimicrobial agents such as NO (i.e., amastigotes) has never been investigated. In the present study, we demonstrated that both axenically grown and intracellular L. amazonensis amastigotes, when submitted to NO action, commit cell suicide through a pathway that is referred to as apoptosis.
In the last few years it has been established that protease activation—in cysteine proteases of the caspase family in particular—is an integral component of the apoptotic cell death metabolic pathway (64). We report here that cell-permeable caspase inhibitors had no effect on the oligonucleosomal DNA fragmentation induced by NO on L. amazonensis amastigotes. This result correlated with observations made on T. brucei brucei exposed to reactive oxygen species (48). The inhibitors used are known, at least, to block caspases that are directly and indirectly responsible for the activation of endonuclease activity (i.e., caspases 3 and 1, respectively). NOS II induction is triggered and regulated by a series of signaling pathways inducing NF-κB (56). Inhibitors of the proteasome and caspase 3 abrogate the induction of NOS II in macrophages by blocking activation of the transcription factor NF-κB (9, 23). These inhibitors cannot be used in culture models relying on NOS II induction. Thus, our experiments of inhibition were restricted to axenically grown amastigotes. Surprisingly and in contrast to data obtained from African trypanosomes by Ridgley et al. (48), specific irreversible proteasome inhibitors (lactacystin and calpain inhibitor I) markedly inhibited the NO-induced oligonucleosomal DNA fragmentation of Leishmania extracellular amastigotes, suggesting that an NO-mediated apoptosis-like process needed proteasome activity to work. This divergence with the observations made by Ridgley et al. (48) might be explained by the status of cell maturation of the parasite considered (35). Moreover, evidence is now accumulating that noncaspases, including cathepsins, calpains, granzymes, and the proteasome complex, also have roles in mediating and promoting cell death (27, 43). Recent studies have otherwise pointed out in two Leishmania species the existence of an active proteasome, one similar to the proteasomes of other eukaryotes (10, 49). This potent proteasome might be involved in the NO-mediated apoptosis-like process. This finding suggests the existence of an ancestral noncaspase protease cell death pathway.
Finally, NO-mediated cytostatic and cytotoxic effects on axenically grown amastigotes were thus a consequence of the activation of a cell death program exhibiting features of apoptosis. A proteasome-dependent apoptosis-like machinery is also present and active in the clinically relevant stage of the parasite (i.e., amastigote) and can be induced in the host cell by the l-arginine-NO pathway. However, this apoptotic event might limit the host's inflammatory and specific immune responses, leading to a decrease in NO-dependent parasite killing and favoring the persistence of some parasites. Nevertheless, NO donors have been used to cure murine and human cutaneous leishmaniasis (15, 65). The investigation of an apoptotic process in cells from NO-treated lesions might merit further research.
Acknowledgments
We thank Phil Agnew for reviewing the manuscript and for many useful suggestions.
Editor: B. B. Finlay
REFERENCES
- 1.Albina, J. E., and J. S. Reichner. 1998. Role of nitric oxide in mediation of macrophage cytotoxicity and apoptosis. Cancer Metastasis Rev. 17:39-53. [DOI] [PubMed] [Google Scholar]
- 2.Albina, J. E., S. Cui, R. B. Mateo, and J. S. Reichner. 1993. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J. Immunol. 150:5080-5085. [PubMed] [Google Scholar]
- 3.Ameisen, J. C., T. Idzioreck, O. Billaut-Mulot, M. Loyens, J. P. Tissier, A. Potentier, and A. Ouaissi. 1995. Apoptosis in a unicellular eukaryote (Trypanosoma cruzi): implications for the evolutionary origin and the control of programmed cell death in the control of cell proliferation, differentiation and survival. Cell. Death Differ. 2:285-300. [PubMed] [Google Scholar]
- 4.Ameisen, J. C. 1996. The origin of programmed cell death. Science 272:1278-1279. [DOI] [PubMed] [Google Scholar]
- 5.Bates, P. A. 1993. Characterization of developmentally regulated nucleases in promastigotes and amastigotes of Leishmania mexicana. FEMS Microbiol. Lett. 107:53-58. [DOI] [PubMed] [Google Scholar]
- 6.Bates, P. A., C. D. Robertson, L. Tetley, and G. H. Coombs. 1992. Axenic cultivation and characterization of Leishmania mexicana amastigote-like forms. Parasitology 105:193-202. [DOI] [PubMed] [Google Scholar]
- 7.Berman, J. D., J. V. Gallalee, and J. M. Best. 1987. Sodium stibogluconate (Pentostam) inhibition of glucose catabolism via the glycolytic pathway, and fatty acid beta-oxidation in Leishmania mexicana amastigotes. Biochem. Pharmacol. 36:197-201. [DOI] [PubMed] [Google Scholar]
- 8.Billaut-Mulot, O., R. Fernandez-Gomez, M. Loyens, and A. Ouaissi. 1996. Trypanosoma cruzi elongation factor 1-alpha: nuclear localization in parasites undergoing apoptosis. Gene 174:19-26. [DOI] [PubMed] [Google Scholar]
- 9.Chakravortty, D., Y. Kato, T. Sugiyama, N. Koide, M. M. Mu, T. Yoshida, and T. Yokoshi. 2001. Inhibition of caspase-3 abrogates lipopolysaccharide-induced nitric oxide production by preventing activation of NF-κB and c-jun NH2-terminal kinase/stress-activated protein kinase in RAW 264.7 murine macrophage cells. Infect. Immun. 69:1315-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen, C. B., L. Jorgensen, A. T. Jensen, S. Gasim, M. Chen, A. Kharazmi, T. G. Theander, and K. Andresen. 2000. Molecular characterization of a Leishmania donovani cDNA clone with similarity to human 20S proteasome a-type subunit. Biochim. Biophys. Acta 1500:77-87. [DOI] [PubMed] [Google Scholar]
- 11.Cornillon, S., C. Foa, J. Davoust, N. Buonavista, J. D. Gross, and P. Golstein. 1994. Programmed cell death in Dictyostelium. J. Cell Sci. 107:2691-2704. [DOI] [PubMed] [Google Scholar]
- 12.Cui, S., J. S. Reichner, R. B. Mateo, and J. E. Albina. 1994. Activated murine macrophages induce apoptosis in tumor cells through nitric oxide-dependent or -independent mechanisms. Cancer Res. 54:2462-2467. [PubMed] [Google Scholar]
- 13.Cysne-Finkelstein, L., R. M. Temporal, F. A. Alves, and L. L. Leon. 1998. Leishmania amazonensis: long-term cultivation of axenic amastigotes is associated to metacyclogenesis of promastigotes. Exp. Parasitol. 89:58-62. [DOI] [PubMed] [Google Scholar]
- 14.Das, L., N. Datta, S. Bandyopadhyay, and P. K. Das. 2001. Successful therapy of lethal murine visceral leishmaniasis with cystatin involves upregulation of nitric oxide and a favorable T-cell response. J. Immunol. 166:4020-4028. [DOI] [PubMed] [Google Scholar]
- 15.Davidson, R. N., V. Yardley, S. L. Croft, P. Konecny, and N. Benjamin. 2000. A topical nitric oxide-generating therapy for cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 94:319-322. [DOI] [PubMed] [Google Scholar]
- 16.Duhe, R. J., M. D. Nielsen, A. H. Dittman, E. C. Villacres, E. J. Choi, and D. R. Storm. 1994. Oxidation of critical cysteine residues of type I adenyl cyclase by o-iodosobenzoate or nitric oxide reversibly inhibits stimulation by calcium and calmodulin. J. Biol. Chem. 269:7290-7296. [PubMed] [Google Scholar]
- 17.Evans, T. G., L. Thai, D. L. Granger, and J. B., Jr. Hibbs. 1993. Effect of in vivo inhibition of nitric oxide production in murine leishmaniasis. J. Immunol. 151:907-915. [PubMed] [Google Scholar]
- 18.Fortenberry, J. D., M. L. Owens, M. R. Brown, D. Atkinson, and L. A. S. Brown. 1998. Exogenous nitric oxide enhance neutrophil cell death and DNA fragmentation. Ann. J. Respir. Cell Mol. Biol. 18:421-428. [DOI] [PubMed] [Google Scholar]
- 19.Girard, P., and P. Potier. 1993. NO, thiols, and disulfides. FEBS Lett. 320:7-8. [DOI] [PubMed] [Google Scholar]
- 20.Gobert, A. P., S. Semballa, S. Daulouede, S. Lesthelle, M. Taxile, B. Veyret, and P. Vincendeau. 1998. Murine macrophages use oxygen- and nitric oxide-dependent mechanisms to synthesize S-nitroso-albumin and to kill extracellular trypanosomes. Infect. Immun. 66:4068-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green, D., and G. Kroemer. 1998. The central executioners of apoptosis: caspases or mitochondria? Trends Cell. Biol. 8:267-271. [DOI] [PubMed] [Google Scholar]
- 22.Green, S. J., M. S. Meltzer, J. B. Hibbs, Jr., and C. A. Nacy. 1990. Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J. Immunol. 144:278-283. [PubMed] [Google Scholar]
- 23.Griscavage, J. M., S. Wilk, and L. J. Ignarro. 1996. Inhibitors of the proteasome pathway interfere with induction of nitric oxide synthase in macrophages by blocking activation of transcription factor NF-κB. Proc. Natl. Acad. Sci. USA 93:3308-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodgkinson, V. H., L. Soong, S. M. Duboise, and D. McMahon-Pratt. 1996. Leishmania amazonensis: cultivation and characterization of axenic amastigote-like organisms. Exp. Parasitol. 83:94-105. [DOI] [PubMed] [Google Scholar]
- 25.Idziorek, T., J. Estaquier, F. De Bels, and J. C. Ameisen. 1995. YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability. J. Immunol. Methods 185:249-258. [DOI] [PubMed] [Google Scholar]
- 26.James, S. L., and C. Nacy. 1993. Effector functions of activated macrophages against parasites. Curr. Opin. Immunol. 5:518-523. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, D. E. 2000. Noncaspase proteases in apoptosis. Leukemia 14:1695-1703. [DOI] [PubMed] [Google Scholar]
- 28.Lancaster, J. R. 1996. Diffusion of free nitric oxide. Methods Enzymol. 268:31-50. [DOI] [PubMed] [Google Scholar]
- 29.Lemesre, J. L., D. Sereno, S. Daulouède, B. Veyret, N. Brajon, and P. Vincendeau. 1997. Leishmania spp.: nitric oxide-mediated metabolic inhibition of promastigote and axenically grown amastigote forms. Exp. Parasitol. 86:58-68. [DOI] [PubMed] [Google Scholar]
- 30.Lemesre, J. L., M. P. Blanc, P. Grebaut, V. Zilberfarb, and V. Carrière. 1994. Culture continue des formes amastigotes de leishmanies en condition axénique: réalisation du cycle évolutif in vitro. Med. Armee 22:99-100. [Google Scholar]
- 31.Lepoivre, M., J. M. Flaman, P. Bobe, G. Lemaire, and Y. Henry. 1994. Quenching of the tyrosyl free radical of ribonucleotide reductase by nitric oxide: relationship to cytostasis induced in tumor cells by cytotoxic macrophages. J. Biol. Chem. 269:21891-21897. [PubMed] [Google Scholar]
- 32.Mauel, J., and A. Ransijn. 1997. Leishmania spp.: mechanisms of toxicity of nitrogen oxidation products. Exp. Parasitol. 87:98-111. [DOI] [PubMed] [Google Scholar]
- 33.Mauel, J., A. Ransijn, and Y. Buchmuller-Rouiller. 1991. Killing of Leishmania parasites in activated murine macrophages is based on an l-arginine-dependent process that produces nitrogen derivatives. J. Leukoc. Biol. 49:73-82. [DOI] [PubMed] [Google Scholar]
- 34.Melino, G., F. Bernassola, R. A. Knight, M. T. Corasaniti, G. Nistico, and A. Finazzi-Agro. 1997. S-nitrosylation regulates apoptosis. Nature 388:432-433. [DOI] [PubMed] [Google Scholar]
- 35.Meriin, A. B., V. L. Gabai, J. Yaglom, V. I. Shifrin, and M. Y. Sherman. 1998. Proteasome inhibitors activate stress kinases and induce Hsp72: diverse effects on apoptosis. J. Biol. Chem. 273:6373-6379. [DOI] [PubMed] [Google Scholar]
- 36.Messmer, U. K., U. K. Reed, and B. Brune. 1996. Bcl-2 protects macrophages from nitric oxide-induced apoptosis. J. Biol. Chem. 271:20192-20197. [DOI] [PubMed] [Google Scholar]
- 37.Mittra, B., A. Saha, A. R. Chowdhury, C. Pal, S. Mandal, S. Mukhopadhyay, S. Bandyopadhyay, and H. K. Majumder. 2000. Luteolin, an abundant dietary component is a potent antileishmanial agent that acts by inducing topoisomerase II-mediated kinetoplast DNA cleavage leading to apoptosis. Mol. Med. 6:527-541. [PMC free article] [PubMed] [Google Scholar]
- 38.Mnaimneh, S., M. Geffard, B. Veyret, and P. Vincendeau. 1997. Albumin nitrosylated by activated macrophages possesses antiparasitic effects neutralized by anti-NO-acetylated-cysteine antibodies. J. Immunol. 158:308-314. [PubMed] [Google Scholar]
- 39.Moreira, M. E., H. A. Del Portillo, R. V. Milder, J. M. Balanco, and M. A. Barcinski. 1996. Heat shock induction of apoptosis in promastigotes of the unicellular organism Leishmania (Leishmania) amazonensis. J. Cell Physiol. 167:305-313. [DOI] [PubMed] [Google Scholar]
- 40.Mossalayi, M. D., M. Arock, D. Mazier, P. Vincendeau, and I. Vouldoukis. 1999. The human immune response during cutaneous leishmaniasis: NO problem. Parasitol. Today 15:342-345. [DOI] [PubMed] [Google Scholar]
- 41.Muhl, H., K. Sandau, B. Brune, V. A. Briner, and J. Pfeilschifter. 1996. Nitric oxide donors induce apoptosis in glomerular mesangial cells, epithelial cells and endothelial cells. Eur. J. Pharmacol. 317:137-149. [DOI] [PubMed] [Google Scholar]
- 42.Nomura, Y., T. Uehara, and M. Nakazawa. 1996. Neuronal apoptosis by glial NO: involvement of inhibition of glyceraldehyde-3-phosphate dehydrogenase. Hum. Cell 9:205-214. [PubMed] [Google Scholar]
- 43.Orlowski, R. Z. 1999. The role of the ubiquitin-proteasome pathway in apoptosis. Cell. Death Differ. 6:303-313. [DOI] [PubMed] [Google Scholar]
- 44.Panaro, M. A., A. Acquafredda, S. Lisi, D. D. Lofrumento, T. Trotta, R. Satalino, V. Mitolo, and O. Brandonisio. 1999. Inducible nitric oxide synthase and nitric oxide production in Leishmania infantum-infected human macrophages stimulated with interferon-gamma and bacterial lipopolysaccharide. Int. J. Clin. Lab. Res. 29:122-127. [DOI] [PubMed] [Google Scholar]
- 45.Picot, S., J. Burnod, V. Bracchi, B. F. Chumpitazi, and P. Ambroise-Thomas. 1997. Apoptosis related to chloroquine sensitivity of the human malaria parasite Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 91:590-591. [DOI] [PubMed] [Google Scholar]
- 46.Pinelli, E., D. Gebhard, A. M. Mommaas, M. van Hoeij, J. A. Langermans, E. J. Ruitenberg, and V. P. Rutten. 2000. Infection of a canine macrophage cell line with Leishmania infantum: determination of nitric oxide production and antileishmanial activity. Vet. Parasitol. 92:181-189. [DOI] [PubMed] [Google Scholar]
- 47.Ramos, M. G., F. L. A. Rabelo, T. Duarte, R. T. Gazzinelli, and J. I. Alvarez-Leite. 2002. Butyrate induces apoptosis in murine macrophages via caspase-3, but independent of autochrine synthesis of tumor necrosis factor and nitric oxide. Braz. J. Med. Biol. Res. 35:161-173. [DOI] [PubMed] [Google Scholar]
- 48.Ridgley, E. L., Z. H. Xiong, and L. Ruben. 1999. Reactive oxygen species activate a Ca2+-dependent cell death pathway in the unicellular organism Trypanosoma brucei brucei. Biochem. J. 340:33-40. [PMC free article] [PubMed] [Google Scholar]
- 49.Robertson, C. D. 1999. The Leishmania mexicana proteasome. Mol. Biochem. Parasitol. 103:49-60. [DOI] [PubMed] [Google Scholar]
- 50.Roy, J. B., and R. G. Wilkerson. 1984. Fallibility of Griess (nitrite) test. Urology 23:270-271. [PubMed] [Google Scholar]
- 51.Salvati, L., M. Mattu, M. Colasanti, A. Scalone, G. Venturini, L. Gradoni, and P. Ascenzi. 2001. NO donors inhibit Leishmania infantum cysteine proteinase activity. Biochim. Biophys. Acta 1545:357-366. [DOI] [PubMed] [Google Scholar]
- 52.Scott, P. 1991. IFN-γ modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J. Immunol. 147:3149-3155. [PubMed] [Google Scholar]
- 53.Sereno, D., and J. L. Lemesre. 1997. Axenically cultured amastigote forms as an in vitro model for investigation of antileishmanial agents. Antimicrob. Agents Chemother. 41:972-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sereno, D., P. Holzmuller, I. Mangot, G. Cuny, A. Ouaissi, J. L. Lemesre. 2001. Antimonial-mediated DNA fragmentation in Leishmania infantum amastigotes. Antimicrob. Agents Chemother. 45:2064-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sisto, M., O. Brandonisio, M. A. Panaro, M. A., A. Acquafredda, A., D. Leogrande, A. Fasanella, T. Trotta, L. Fumarola, and V. Mitolo. 2001. Indicible nitric oxide synthase expression in Leishmania-infected dog macrophages. Comp. Immunol. Microbiol. Infect. 24:247-254. [DOI] [PubMed] [Google Scholar]
- 56.Tsai, S. H., S. Y. Lin-Shiau, and J. K. Lin. 1999. Suppression of nitric oxide synthase and the downregulation of the activation of NFκB in macrophages by resveratrol. Br. J. Pharmacol. 126:673-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vincendeau, P., S. Daulouede, B. Veyret, M. L. Darde, B. Bouteille, and J. L. Lemesre. 1992. Nitric oxide-mediated cytostatic activity on Trypanosoma brucei gambiense and Trypanosoma brucei brucei. Exp. Parasitol. 75:353-360. [DOI] [PubMed] [Google Scholar]
- 58.Vouldoukis, I., P. A. Becherel, V. Riveros-Moreno, M. Arock, O. da Silva, P. Debre, D. Mazier, and M. D. Mossalayi. 1997. Interleukin-10 and interleukin-4 inhibit intracellular killing of Leishmania infantum and Leishmania major by human macrophages by decreasing nitric oxide generation. Eur. J. Immunol. 27:860-865. [DOI] [PubMed] [Google Scholar]
- 59.Vouldoukis, I., J. C. Drapier, A. K. Nussler, Y. Tselentis, O. A. Da Silva, M. Gentilini, D. M. Mossalayi, L. Monjour, and B. Dugas. 1996. Canine visceral leishmaniasis: successful chemotherapy induces macrophage antileishmanial activity via the l-arginine nitric oxide pathway. Antimicrob. Agents Chemother. 40:253-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vouldoukis, I., V. Riveros-Moreno, B. Dugas, F. Ouaaz, P. Becherel, P. Debre, S. Moncada, and M. D. Mossalayi. 1995. The killing of Leishmania major by human macrophages is mediated by nitric oxide induced after ligation of the Fc epsilon RII/CD23 surface antigen. Proc. Natl. Acad. Sci. USA 92:7804-7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welburn, S. C., and N. B. Murphy. 1998. Prohibitin and RACK homologues are upregulated in trypanosomes induced to undergo apoptosis and in naturally occurring terminally differentiated forms. Cell. Death Differ. 5:615-622. [DOI] [PubMed] [Google Scholar]
- 62.Welburn, S. C., S. Lillico, and N. B. Murphy. 1999. Programmed cell death in procyclic form Trypanosoma brucei rhodesiense: identification of differentially expressed genes during Con A-induced death. Mem. Inst. Oswaldo Cruz 94:229-234. [DOI] [PubMed] [Google Scholar]
- 63.Xie, K., Y. Wang, S. Huang, L. Xu, D. Bielenberg, T. Salas, D. J. McConkey, W. Jiang, and I. J. Fidler. 1997. Nitric oxide-mediated apoptosis of K-1735 melanoma cells is associated with downregulation of Bcl-2. Oncogene 15:771-779. [DOI] [PubMed] [Google Scholar]
- 64.Yuan, J., S. Shaham, S. Ledoux, H. M. Ellis, and H. R. Horvitz. 1993. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1β-converting enzyme. Cell 75:641-652. [DOI] [PubMed] [Google Scholar]
- 65.Zeina, B., C. Banfield, and S. Al-Assad. 1997. Topical glyceryl trinitrate: a possible treatment for cutaneous leishmaniasis. Clin. Exp. Dermatol. 22:244-245. [PubMed] [Google Scholar]