Abstract
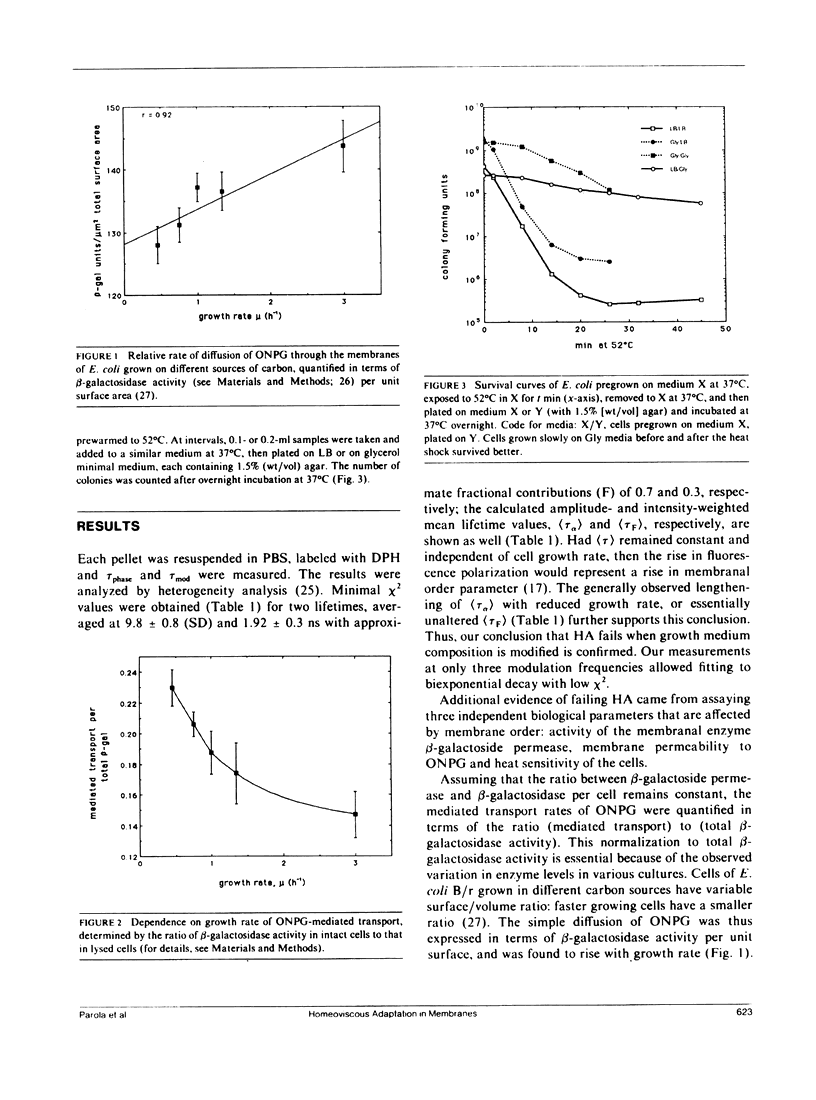
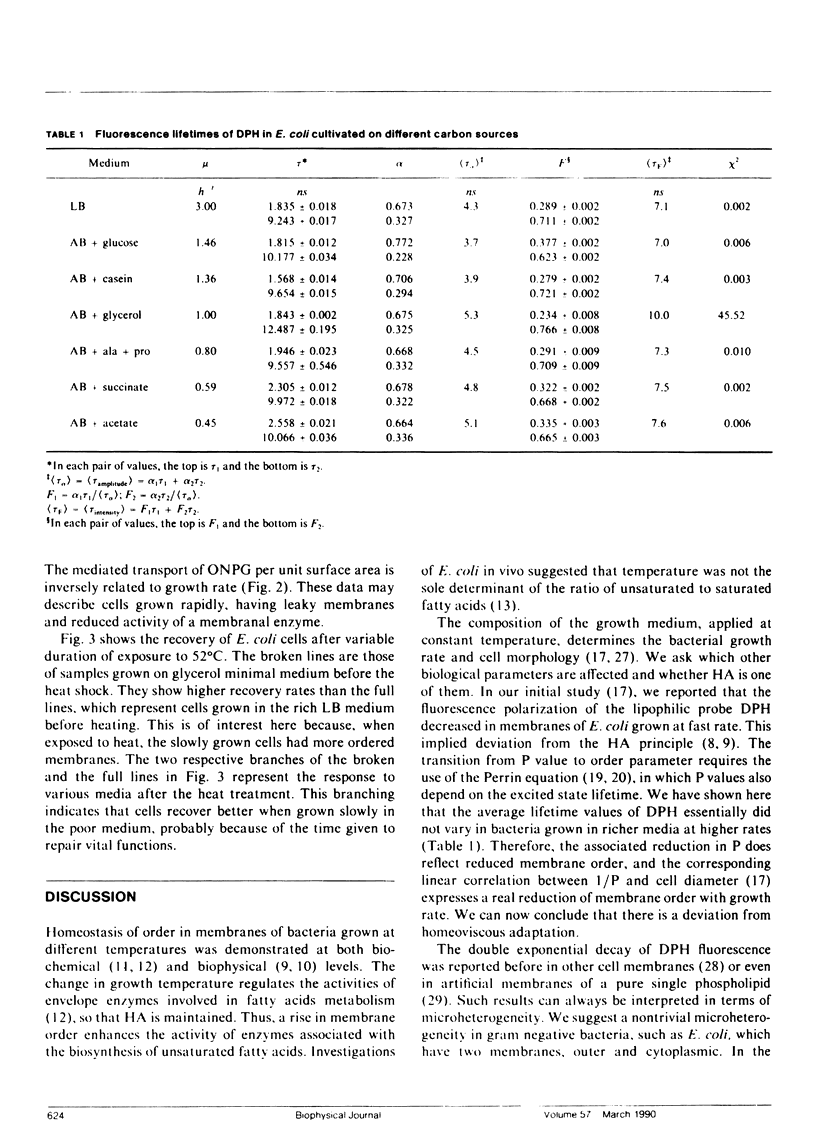
The process by which an organism changes the composition of its membranal fatty acids in response to growth temperature, so as to maintain optimal membrane functioning, is known as homeoviscous adaptation (HA). One expression of HA is the constancy of the fluorescence polarization (P) of the lipophilic probe 1,6-diphenyl-1,3,5-hexatriene (DPH) in membranes of cells grown at various temperatures. The P of DPH in the membranes of Escherichia coli was shown by us to be inversely proportional to bacterial growth rate on different carbon sources. This result, implying failure of HA, is now complemented by measurements of DPH lifetimes, which indicate that the dominant variables contributing to the drop in P are (a) the order parameter of the membrane, which goes down, and (b) the fluidity, which may slightly increase. These are then the changes induced by enhanced growth rate. Two additional effects, cell membrane permeability and sensitivity to thermal shock, determined by the diffusion of o-nitrophenylgalactoside (ONPG) and by exposure to 52 degrees C, respectively, are reported to increase with growth rate. We can now conclude that there is a deviation from the principle of HA in E. coli grown at various rates, brought about by controlling the growth media at constant temperatures.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow D. A., Lentz B. R. Membrane structural domains. Resolution limits using diphenylhexatriene fluorescence decay. Biophys J. 1985 Aug;48(2):221–234. doi: 10.1016/S0006-3495(85)83775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borochov H., Shinitzky M. Vertical displacement of membrane proteins mediated by changes in microviscosity. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4526–4530. doi: 10.1073/pnas.73.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHO K. Y., SALTON M. R. FATTY ACID COMPOSITION OF THE LIPIDS OF MEMBRANES OF GRAM-POSITIVE BACTERIA AND "WALLS" OF GRAM-NEGATIVE BACTERIA. Biochim Biophys Acta. 1964 Dec 2;84:773–775. doi: 10.1016/0926-6542(64)90043-5. [DOI] [PubMed] [Google Scholar]
- Chan M., Himes R. H., Akagi J. M. Fatty acid composition of thermophilic, mesophilic, and psychrophilic clostridia. J Bacteriol. 1971 Jun;106(3):876–881. doi: 10.1128/jb.106.3.876-881.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossins A. R., Behan M., Jones G., Bowler K. Lipid-protein interactions in the adaptive regulation of membrane function. Biochem Soc Trans. 1987 Feb;15(1):77–81. doi: 10.1042/bst0150077. [DOI] [PubMed] [Google Scholar]
- D'Agnolo G., Rosenfeld I. S., Vagelos P. R. Multiple forms of beta-ketoacyl-acyl carrier protein synthetase in Escherichia coli. J Biol Chem. 1975 Jul 25;250(14):5289–5294. [PubMed] [Google Scholar]
- Davis M. T., Silbert D. F. Changes in cell permeability following a marked reduction of saturated fatty acid content of Escherichia coli K-12. Biochim Biophys Acta. 1974 Dec 10;373(2):224–241. doi: 10.1016/0005-2736(74)90147-3. [DOI] [PubMed] [Google Scholar]
- Dennis W. H., Yatvin M. B. Correlation of hyperthermic sensitivity and membrane microviscosity in E. coli K1060. Int J Radiat Biol Relat Stud Phys Chem Med. 1981 Mar;39(3):265–271. doi: 10.1080/09553008114550341. [DOI] [PubMed] [Google Scholar]
- Eze M. O., McElhaney R. N. The effect of alterations in the fluidity and phase state of the membrane lipids on the passive permeation and facilitated diffusion of glycerol in Escherichia coli. J Gen Microbiol. 1981 Jun;124(2):299–307. doi: 10.1099/00221287-124-2-299. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Prendergast F. G., Hogen D. Fluorescence anisotropy measurements under oxygen quenching conditions as a method to quantify the depolarizing rotations of fluorophores. Application to diphenylhexatriene in isotropic solvents and in lipid bilayers. Biochemistry. 1979 Feb 6;18(3):520–527. doi: 10.1021/bi00570a022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porat N., Gill D., Parola A. H. Adenosine deaminase in cell transformation. Biophysical manifestation of membrane dynamics. J Biol Chem. 1988 Oct 15;263(29):14608–14611. [PubMed] [Google Scholar]
- Quinn P. J. The fluidity of cell membranes and its regulation. Prog Biophys Mol Biol. 1981;38(1):1–104. doi: 10.1016/0079-6107(81)90011-0. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Alon N., Buchwald M. Plasma membrane lipids of human diploid fibroblasts from normal individuals and patients with cystic fibrosis. Biochim Biophys Acta. 1979 Jul 27;574(1):39–47. doi: 10.1016/0005-2760(79)90082-1. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- Shen P. Y., Coles E., Foote J. L., Stenesh J. Fatty acid distribution in mesophilic and thermophilic strains of the genus Bacillus. J Bacteriol. 1970 Aug;103(2):479–481. doi: 10.1128/jb.103.2.479-481.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M., Pinkerton F., Sutherland E., Simon F. R. Rate limitation of (Na+ + K+)-stimulated adenosinetriphosphatase by membrane acyl chain ordering. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4893–4897. doi: 10.1073/pnas.76.10.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Parola A. H., Abdah M., Masalha H. Homeoviscous adaptation, growth rate, and morphogenesis in bacteria. Biophys J. 1985 Aug;48(2):337–339. doi: 10.1016/S0006-3495(85)83788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Woldringh C. L., Mirelman D. Constant peptidoglycan density in the sacculus of Escherichia coli B/r growing at different rates. FEBS Lett. 1979 Feb 1;98(1):29–32. doi: 10.1016/0014-5793(79)80144-1. [DOI] [PubMed] [Google Scholar]


