Abstract
Bovine paratuberculosis is caused by the infection of young calves with Mycobacterium avium subsp. paratuberculosis, resulting in a chronic granulomatous infection of predominantly the ileum. After an incubation period of 2 to 5 years, the disease becomes progressive in some of the chronically infected, but asymptomatic cows. This results in a protein-losing enteropathy that will ultimately be fatal. A loss of cell-mediated immune responses in symptomatic animals has been described, but no information is available concerning immune reactivity in the intestine. We sought to investigate putative disease status-associated lymphocyte subset distributions and antigen-specific functional characteristics of mononuclear cells isolated from blood, gut-associated lymphoid tissue, and the intestinal walls of 22 cows in different stages of disease and in control animals. The results demonstrated a significant decrease in CD4+ T-cell frequency and a significant increase in TcR1-N12+ γδ T-cell frequency in ileum lamina propria lymphocytes of symptomatic animals compared to the asymptomatic shedders. Immunohistology revealed that there was also an absolute decrease in the number of CD4+ T cells in sections of the lesional ileum. Our findings also indicated that both peripheral and intestinal cell-mediated responses are decreased in symptomatic animals compared to asymptomatic animals. We conclude that the decrease in cell-mediated responses is likely related to a loss of antigen-specific CD4+ T cells, which is most prominent in the lesional ileum from symptomatic animals, thus contributing to the progressive nature of bovine paratuberculosis.
Bovine paratuberculosis is an intestinal mycobacteriosis caused by the infection of young calves with Mycobacterium avium subsp. paratuberculosis, resulting in a chronic granulomatous infection of predominantly the ileum. One of the characteristics of the pathogenesis of bovine paratuberculosis is the progression from the prolonged asymptomatic stage (2 to 5 years after infection) to the stage in which clinical signs of protein-losing enteropathy (i.e., diarrhea and weight loss), which will ultimately be fatal, become apparent. This advance to the clinical stage is associated with extensive progression of the granulomatous infection in the small intestine (13, 14). In sheep the clinical stage can be associated with either tuberculoid (granulomas with lymphoid cell infiltrate, low numbers of macrophages with low intracellular bacterial burden) or lepromatous (diffuse and extensive lesions with predominantly macrophage infiltrate with high intracellular bacterial burden) lesions. In bovine paratuberculosis, lesions are mostly of the lepromatous type (8, 14). Observations on the immunological aspects of bovine paratuberculosis have largely been derived from studies aimed at improving immunodiagnostics and have mostly focused on immunological parameters in peripheral blood. From these studies, it became apparent that the progression from asymptomatic to clinical Johne's disease is associated with a decrease in peripheral cell-mediated immunity and increasing production of antibodies (10).
Based on their function and cytokine profiles, T cells can be characterized as Th1 or Th2 T cells (1, 39). Th1 T cells are involved in effective immune responses to intracellular pathogens by promoting microbicidal activities of macrophages via gamma interferon. Th2 T cells promote immunoglobulin G1 (IgG1) antibody production, suppress Th1 activity, and are likely ineffective in case of intracellular infections (1, 7). The Th cell paradigm is not as clear in outbred species, such as cattle, where typical Th1 and Th2 T cells can be found but are outnumbered by the Th0 phenotype T cells (7).
Since it has become clear that by secretion of Th1 and Th2 cytokines by cells other than the CD4+ Th cells play a role in this balance of immune reactivity, Bloom et al. (5) have introduced the terminology of type I and type II responses to encompass the overall response to a complex antigen or pathogen. For bovine paratuberculosis, it may be hypothesized that the change from predominantly cell-mediated immune responses to increased humoral responses is caused by a switch from type I to type II immune reactivity. In addition to data available from the literature, which are reviewed by Chiodini (10), we addressed several aspects of this putative type-I-to-type-II shift in bovine paratuberculosis. In the clinical stage of paratuberculosis, the decrease in peripheral cell-mediated responses to several mycobacterial antigens can be clearly demonstrated (28). However, the distribution of isotypes in antigen-specific antibody responses showed a predominant loss of Th1-associated antibodies (IgG2) rather than an increase in Th2-associated IgG1 (27). To date, no information is available on the local cell-mediated immune responses in the different stages of bovine paratuberculosis.
The aim of the present study was to determine whether changes in local and peripheral T-lymphocyte subsets and cell-mediated immune responses could be observed that were associated with the progression of bovine paratuberculosis from the asymptomatic stage to the clinical stage of the disease. For this purpose, peripheral blood and intestinal lymphocyte populations of healthy animals, vaccinated animals, asymptomatic animals, and animals with clinical Johne's disease were examined by flow cytometry and in situ immunohistochemistry. Functional aspects of blood, mesenteric lymph node, and intestinal lymphocyte populations were determined by antigen-specific and mitogen-induced lymphoproliferation.
MATERIALS AND METHODS
Animals.
Samples were taken from 23 cows (aged 2 to 8 years, average, 3.9 years), which were grouped according to their paratuberculosis status. Samples from five animals were taken at a slaughterhouse in an area of low apparent prevalence of paratuberculosis (control). Apart from the animals in the control group, the cows (n = 18) originated from Dutch dairy farms known to have endemic paratuberculosis. Five cows, which were vaccinated in the first month of life with the M. avium subsp. paratuberculosis strain 3+5/C vaccine (ID-Lelystad, Leylstad, The Netherlands) prepared according to the Office International des Epizooties (OIE) manual (22) (vaccinated, n = 5). A group of asymptomatic shedders (shedders, n = 7) was selected based on prior fecal culture results. Cows with symptoms of clinical disease (clinical, n = 6) were selected based on a history of weight loss, decreased milk production and diarrhea; cachetic animals in advanced clinical stages of paratuberculosis with total serum protein concentrations below 40 mg/ml were excluded. All animals were sampled before and after slaughter.
Sampling.
Blood was taken from the jugular vein by using heparinized tubes prior to slaughter. After exsanguination, a small part of the terminal ileum (ca. 40 cm2) and the draining ileocecal lymph node, as well as a part of distal jejunum (ca. 40 cm2) and the draining jejunal lymph node (both located ca. 2 m proximal of the ileocecal valve), were excised, and carefully freed of mesentery and fat. The lymph nodes were aseptically divided in two equal parts, and one part was stored in RPMI 1640 tissue culture medium supplemented with 2% fetal calf serum (FCS), 50 IU of penicillin/ml, and 50 μg of streptomycin/ml (all from Gibco-BRL, Paisley, United Kingdom) and 5 IU of heparin (Leo Pharmaceutical Products, Weesp, The Netherlands)/ml (wash medium). The other part was again divided in two; one part was stored in a buffered formalin (4%) solution, and the other part, to be used for immunohistochemistry on cryostat sections, was immediately frozen in isopentane cooled by liquid nitrogen. Small parts of the intestinal sections were also processed for later histological examinations as described above. The remainder of the intestinal sections was washed once in phosphate-buffered saline (PBS) to remove mucus and fecal matter and then stored in PBS without Ca/Mg (PBS-0). During transport to the laboratory, the samples were kept on ice except for the cryostat sections, which were transported in liquid nitrogen, and the sections in formalin, which were transported at ambient temperature.
Isolation of lymphocytes.
The lymph nodes were divided into two parts. One part was used for bacteriological culture. The remaining part was cut into pieces of ca. 3 mm3 which were forced through a nylon mesh (NPBI) to obtain a single cell suspension diluted in wash medium.
To isolate intraepithelial lymphocytes (IEL) and lamina propria lymphocytes (LPL) from the sections of small intestine, nonenzymatic mechanical methods were used. The IEL were collected by slow rotation of the intestinal sections (40 cm2) in 35 ml of PBS-0 for 15 min at room temperature three consecutive times. Between treatments, the fluid was aseptically collected and replaced with fresh PBS-0. After the third time the remaining intestinal tissue was used to isolate the LPL (see below). The crude cell suspension containing the IEL was put on ice twice for 10 min each time to allow large particles to sedimentate, after which the IEL containing supernatant was collected, and the pellet was discarded. Finally, the cell suspension was centrifuged for 10 min at 500 × g, and the pellet containing the IEL was resuspended in RPMI 1640 tissue culture medium supplemented with 2% FCS, 50 IU of penicillin/ml, and 50 μg of streptomycin/ml (all from Gibco-BRL) and 5 IU of heparin (Leo Pharmaceutical Products)/ml (wash medium) and stored at 4°C until further processing.
The intestinal sections from which the IEL had been isolated were washed twice in 35 ml of PBS-0 to remove residual IEL. Subsequently, the remaining mucosa was removed by gently scraping the sections, followed by one more wash with 35 ml of PBS-0. At this point samples were taken for routine histology to verify that the IEL-containing mucosa was adequately removed. The serosa was then carefully removed, and the remaining tissue was cut into pieces of ca. 1 mm3. These pieces were kept in suspension in RPMI wash buffer by slow rotation for 30 min at room temperature. To isolate the LPL, the supernatant was collected and centrifuged for 10 min at 500 × g, and the pellet was resuspended in wash medium and stored at 4°C until further processing.
The mononuclear lymphocytes from these lymph node cell suspensions, i.e., the IEL- and LPL-containing suspensions, and from the heparinized blood were collected by Ficoll-Isopaque density gradient centrifugation as described earlier (28), resulting in populations of mesenteric lymph node mononuclear cells (MLMC), peripheral blood mononuclear cells (PBMC), IEL, and LPL. The viability of these cell populations was >95% as assessed by trypan blue dye exclusion.
Cells were either stored in cold PBS supplemented with 1% bovine serum albumin and 0.01% sodium azide (fluorescence-activated cell scan [FACS] buffer) and stored at 4°C until staining for flow cytometric analysis or kept in Iskove tissue culture medium supplemented with 10% FCS, 50 IU of penicillin/ml, 50 μg of streptomycin/ml, 2 mM l-glutamine (all from Gibco-BRL), and 5 × 10−5 M β-mercaptoethanol (Flow Laboratories, Ivume, United Kingdom) for functional assays.
Flow cytometric analysis.
Single-color flow cytometric analysis was performed with monoclonal antibodies against bovine lymphocyte markers that have been described earlier (see Table 1), according to procedures described previously (28). Briefly, cells (PBMC, MLMC, IEL, and LPL) were washed twice in FACS buffer, incubated with the first antibody (Table 1) for 30 min at 4°C, washed twice, subsequently incubated with a fluorescein isothiocyanate-labeled goat anti-mouse immunoglobulin antibody (H+L; Becton Dickinson) for 30 min at 4°C, washed once, and collected in 200 μl of FACS buffer prior to analysis. Analysis was done on a flow cytometer (FACSCalibur; Becton Dickinson). A forward-scatter-side-scatter lifegate was used to measure 2,000 to 5,000 lymphocytes per sample. Based on the fluorescence data of the lymphocytes, the results were expressed as the percentage of cells with positive staining relative to a sample stained with an isotype control antibody. The CD4/CD8 ratio was calculated by dividing the frequency of CD4+ cells by the frequency of CD8+ cells.
TABLE 1.
Monoclonal antibodies used in flow cytometric analysis
(Immuno)histochemistry.
Formalin-fixed samples were processed routinely for histological examination. Sections were cut and stained with hematoxylin and eosin and by the Ziehl-Neelsen method for the detection of acid-fast organisms.
Serial sections (5 μm) of the frozen ileocecal lymph node and ileum of each animal from the control group, each of the shedders, and each of the animals with clinical signs were cut by using a cryotome onto poly-l-lysine-coated glass slides and allowed to air dry at room temperature for 1 h. Sections were fixed in acetone supplemented with 0.07% H2O2 to block endogenous peroxidase for 10 min and washed with distilled water and PBS containing 0.05% Tween 20 (PBS-Tween) for 5 min each. To prevent nonspecific binding of antibodies, slides were preincubated with PBS supplemented with 10% horse serum for 20 min at room temperature. The primary CACT138A (anti-bovine CD4) and CACT61A (anti-bovine TcR1-N12) (Table 1) polyclonal rabbit anti-M. paratuberculosis antibodies (Dako) were added to the serial sections, followed by incubation for 1 h at room temperature. Slides were washed in PBS-Tween, and biotinylated horse anti-mouse antibodies (Vector) were added to the slides, followed by incubation for 30 min. After washing with PBS-Tween, ABC streptavidin-biotin complex (Vector) was added to the slides. Positive staining was visualized by using 3-amino-9-ethylcarbazole (AEC) (Sigma) as the chromogen. Sections were counterstained in hematoxylin, cleared through tap water and destined water, and mounted by using aquamount (BDH, Poole, United Kingdom)
Immunohistochemically stained cells were counted at a ×400 magnification, i.e., fields of 0.159 mm2. For each ileum tissue section, 10 view fields, equally distributed between the villus and crypt areas, were counted. In the case of the lymph node sections, cells in 10 view fields in the paracortical area were enumerated.
Antigens.
Recombinant M. avium subsp. paratuberculosis Hsp65 and Hsp70 were produced according to methods described in detail earlier (16, 27). The purity of the recombinant Hsp65 and Hsp70 was checked by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and preparations were tested for lipopolysaccharide contamination by Limulus amebocyte assay (Sigma, St. Louis, Mo.). The bovine HSP/HSC70 was supplied by Stressgen.
A purified protein derivative (PPD-P) was prepared from M. avium subsp. paratuberculosis strain 3+5/C culture supernatant according to the OIE manual (22) at the Institute for Animal Health and Science (Lelystad, The Netherlands).
M. avium subsp. paratuberculosis strain 316F was grown at the Institute for Animal Health and Science.
Lymphocyte stimulation test.
Lymphocyte stimulation tests were performed in 96-well microtiter plates (Costar, Cambridge, Mass.) by using 100 μl of the PBMC suspension and 100 μl of antigen per well; all tests were performed in triplicate. The mycobacterial antigens PPD-P, Hsp65, Hsp70, and bovine Hsp70 were used in predetermined optimal concentrations of 10 μg/ml each. Strain 316F bacteria were briefly sonicated, counted, and used in a concentration of 107 CFU/ml. Concanavalin A (ConA) was used as a positive control (2.5 μg/ml), and medium alone was used as a negative control.
Cells were cultured at 37°C and 5% CO2 in a humidified incubator for 3 days. Then, 0.4 μCi of [3H]thymidine (Amersham International) was added to each well, and cells were cultured for an additional 18 h. Subsequently, cells were harvested onto glass fiber filters, and the incorporation of [3H]thymidine was measured by liquid scintillation counting and expressed as the stimulation index (SI), which was calculated by dividing the counts per minute (cpm) of a specific stimulation by the cpm of the medium control.
Diagnosis of paratuberculosis.
Samples from the two mesenteric lymph nodes and the two pieces of small intestine were used for bacterial culture, which was performed at the Institute for Animal Science and Health according to a modification of the method of Jorgensen (25). Samples were checked for bacterial growth every 4 weeks and considered negative if, after a culture period of 6 months, no bacterial growth was observed. Bacterial growth was confirmed to be M. avium subsp. paratuberculosis based on mycobactin dependence of the culture and the confirmation of the presence of the specific IS900 insertion sequence by PCR (45).
Statistical analysis.
The SPSS 7.5 statistical software was used for analysis of the data. Data from the flowcytometric analysis were analyzed with one-way analysis of variance, followed by Bonferroni multiple comparisons. For comparisons of anatomical locations, the data on mesenteric lymph nodes and LPL and IEL populations was combined per location and compared to lymphocyte subset frequencies in blood. For comparison of disease status, data were analyzed separately for each isolated population. Nonparametric tests were used for the comparison of the lymphoproliferation assays of the different groups and conditions. For comparisons of similar anatomical locations between the different groups of animals, the Kruskal-Wallis test was used for one-way analysis of variance. Comparisons between the different anatomical locations within groups of animals were made by using the Friedman test for two-way analysis of variance by ranks. In case of significant differences between groups as indicated by the analysis of variance, multiple comparisons of groups were made according to methods described previously (41). The level of significance was set at P < 0.05.
RESULTS
Diagnosis of paratuberculosis.
The results of bacteriological cultures are summarized in Table 2. No bacterial growth was observed in samples from control animals. In the vaccinated animals, 40% of the ileocecal and jejunal lymph nodes, 60% of the ileal sections, and 80% of the jejunal sections were culture positive. One vaccinated animal tested negative on all samples. Except for the culture of one ileocecal and one jejunal lymph node section of the shedders, all samples were culture positive. In the shedders and vaccinated animals the animals that had been positively identified by fecal culture were also positive for at least two of four tissue sections cultured per animal. All samples from the animals with clinical Johne's disease had a positive bacteriological culture, and histological examinations showed diffuse granulomatous lesions. These lesions were most apparent in the ileum. Ziehl-Neelsen staining showed the presence of macrophages and multinucleated giant cells with high numbers of acid-fast bacteria.
TABLE 2.
Results of bacteriological culture for M. avium subsp. paratuberculosis in tissue sections
| Status | No. of samples positive/total no. cultureda in:
|
|||
|---|---|---|---|---|
| Ileum | Ileocecal LN | Jejunum | Jejunal LN | |
| Control | 0/5 | 0/5 | 0/5 | 0/5 |
| Vaccinated | 3/5 | 2/5 | 4/5 | 2/5 |
| Shedder | 6/6 | 5/6 | 6/6 | 5/6 |
| Clinical | 6/6 | 6/6 | 6/6 | 6/6 |
The numbers indicate the number of samples with growth of M. avium subsp. paratuberculosis/total number of samples cultured. LN, lymph node.
Flow cytometric analysis: lymphocyte subset distributions and anatomical locations.
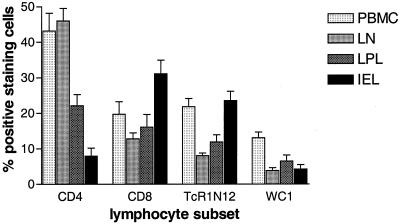
Flow cytometric analysis of CD4+ CD8+ TcR1-N12+ cell subsets, as well as the WC1+ cells, which are a subset of the TcR1-N12+ γδ T cells, indicated differences that were related to the anatomical site from which cells were isolated. In animals from the control group (Fig. 1), the mononuclear cells isolated from the blood and the mesenteric lymph nodes consisted predominantly of CD4+ T cells (43.2 and 46.0%, respectively) and had comparable frequencies of CD8+ T cells (19.8 and 12.9%, respectively). In blood a higher frequency of TcR1-N12+ cells were found compared to in the lymph nodes (21.8 and 8.1%, respectively [P < 0.05]). Compared to blood and lymph nodes, the frequency of CD4+ T cells in the LPL population (22.1%) was significantly lower (P < 0.05 for both comparisons). The frequency of CD8+ T cells was comparable in blood, lymph nodes, and LPL (16.1%). The frequency of TcR1-N12+ γδ T cells in the LPL populations (11.9%) was comparable to the frequency observed in mesenteric lymph nodes but lower than that observed in blood (P < 0.05). The frequency of CD4+ T cells in the IEL populations (7.9%) was significantly lower compared to frequencies in blood, mesenteric lymph nodes, and LPL populations (P < 0.05 for all comparisons). The frequency of CD8+ T cells in the IEL populations (31.1%) was significantly higher compared to frequencies in blood, mesenteric lymph nodes, and LPL populations (P < 0.05 for all comparisons). The frequency of TcR1-N12+ γδ T cells in IEL populations was significantly higher compared to mesenteric lymph node and LPL populations (P < 0.05 for both comparisons) but was comparable to the frequency observed in blood. The frequency of WC1+ γδ T cells (13.1%) was higher in blood compared to frequencies found in mesenteric lymph nodes (3.4% [P < 0.05]), LPL populations (6.5% [P < 0.05]), and IEL populations (4.3% [P < 0.05]).
FIG. 1.
Distribution of lymphocyte subsets related to anatomical location. Representative data on the frequency (percent positive-staining cells in flow cytometry plus the standard error [SE], n = 5 animals [group: control]) of the different lymphocyte subsets (CD4+ T cells, CD8+ T cells, TcR1-N12+ γδ T cells, and WC1+ γδ T cells) in mononuclear cell populations isolated from blood (PBMC), the mesenteric lymph nodes (LN), the lamina propria (LPL), and the epithelium (IEL). WC1+ γδ T cells are a subset of the TcR1-N12+ γδ T cells, most γδ T cells in PBMC are WC1+ in contrast to those in mesenteric lymph nodes, LPL, and IEL. CD4+ T cells predominate in PBMC and mesenteric lymph node populations, but their frequency is very low in IEL.
For these overall differences of lymphocyte subset distributions related to anatomical location, no statistical significant changes were found between the different groups of animals studied (data not shown), indicating the validity of the procedures to isolate lymphocytes from different anatomical locations. The data obtained from the control group is shown as being representative for the differences observed between the different anatomical locations (Fig. 1).
Lymphocyte subset distribution and disease status. (i) CD4+-T-cell frequencies.
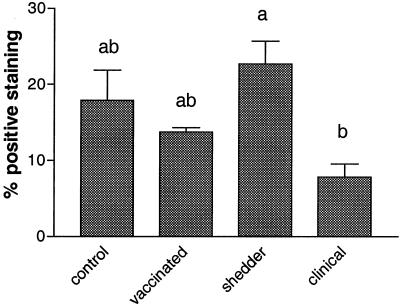
Differences between the frequencies of CD4+ T cells were found in the LPL populations of the ileum when we compared the different groups of animals. Animals with clinical signs of paratuberculosis had significantly fewer CD4+ T cells (7.8% ± 1.9%) compared to shedders (22.7% ± 3.3%) (P < 0.01) (Fig. 2). A similar trend was observed in CD4+-T-cell frequencies of the ileocecal lymph node draining this part of the ileum, but these differences were not statistically significant (Table 3). In the other lymphocyte populations, no significant disease-associated changes were observed.
FIG. 2.
CD4+ αβ T-cell numbers in LPL in ileum sections as determined by FACS analysis. The frequency of CD4+ T cells (plus the SE) in LPL populations isolated from the ileum of cows from the control (n = 5), vaccinated (n = 5), shedder (n = 6), and clinical (n = 6) groups. Letters above columns indicate the results of statistical analysis. Columns that do not share letters are significantly different (P < 0.05).
TABLE 3.
T-lymphocyte subset frequencies and disease statusa
| Location | Avg % positive-staining cells (SE)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CD4+ T cells
|
TcR1-N12+ γδ T cells
|
|||||||
| Control (n = 5) | Vaccinated (n = 5) | Shedder (n = 6) | Clinical (n = 6) | Control (n = 5) | Vaccinated (n = 5) | Shedder (n = 6) | Clinical (n = 6) | |
| PBMC | 43.2 (5.0) | 33.7 (4.6) | 22.0 (3.1) | 21.4 (8.3) | 21.8 (2.4) | 14.6 (1.8) | 4.4 (1.5) | 7.3 (2.1) |
| LN ileum | 43.6 (2.3) | 37.4 (3.7) | 49.9 (7.8) | 26.1 (8.4) | 8.8 (1.3) | 4.2 (0.9) | 2.4 (0.7) | 4.1 (1.4) |
| LN jejunum | 48.5 (7.0) | 41.5 (4.1) | 47.2 (4.7) | 28.6 (8.1) | 7.3 (0.7) | 4.1 (0.4) | 5.8 (2.2) | 5.4 (1.5) |
| LPL ileum | 17.9 (3.9) | 13.8 (0.6) | 22.7 (3.3) | 7.8 (1.9) | 10.5 (2.5) | 14.7 (2.0) | 10.9 (4.2) | 23.9 (6.3) |
| LPL jejunum | 26.3 (4.5) | 18.4 (2.0) | 29.9 (5.2) | 20.8 (9.2) | 13.4 (3.2) | 14.4 (2.0) | 9.9 (4.4) | 24.6 (5.7) |
| IEL ileum | 11.7 (4.0) | 4.9 (2.0) | 8.7 (2.3) | 3.6 (0.9) | 27.6 (3.4) | 19.7 (4.6) | 18.3 (5.4) | 28.1 (4.5) |
| IEL jejunum | 4.3 (0.6) | 7.4 (2.3) | 16.7 (7.7) | 13.7 (8.8) | 19.5 (3.1) | 24.1 (4.1) | 21.0 (8.3) | 24.8 (6.5) |
Data are expressed as the average percentage of cells showing positive staining for the given marker (CD4 and TcR1-N12), location (PBMC, mesenteric lymphnode cells [LN], LPL, and IEL), and disease status.
(ii) CD8+-T-cell frequencies.
The frequencies of CD8+ T cells did not differ when we compared the four different groups of animals.
(iii) TcR1-N12+ γδ T-cell frequencies.
The frequencies of TcR1-N12+ γδ T cells were significantly increased in the ileum LPL populations of animals with clinical signs of paratuberculosis (23.9% ± 6.3%) compared to frequencies observed in shedders (10.9% ± 4.2% [P < 0.05]) and control animals (10.5% ± 2.5% [P < 0.05]). This pattern was not seen in the TcR1-N12 γδ T-cell frequencies in the ileocecal lymph node. In the other lymphocyte populations no significant disease-associated changes were observed (Table 3).
(iv) Other lymphocyte subsets.
No disease status-associated changes were observed with respect to WC1+ γδ T cells, CD14+ monocytes/macrophages, or CD21+ B cells (data not shown).
Immunohistology.
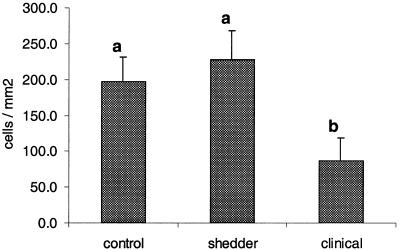
Immunohistological evaluation showed that the numbers of CD4+ cells in the lamina propria of the ilea of animals with clinical signs were lower compared to those observed for shedders and control animals (Fig. 3). Upon comparing control and clinical animals, we noted a significant reduction (P < 0.05) of 55.6% (compared to 56.4% as judged by flow cytometric analysis). Upon comparing CD4+ cells of shedders and clinical animals, we observed a significant reduction (P < 0.05) of 60.0% (compared to 47.7% as judged by flow cytometric analysis). With respect to the ileocecal lymph node sections, the number of CD4+ cells in animals with clinical signs was lower compared to those of shedders and control animals (P < 0.05). Differences in the numbers of TcR1-N12+ cells, as enumerated by immunohistology, did not differ significantly between the different groups of animals. The average TcR1-N12/CD4 ratio in the ilea of animals with clinical signs was 3.1 and was comparable to the average TcR1-N12/CD4 ratio of 2.9 observed with flow cytometry.
FIG. 3.
Numbers of CD4+ αβ T cells in LPL ileum sections. The number of CD4+ αβ T cells per mm2 of lamina proprium (plus the SE), as determined by immunohistochemistry, is shown for sections of ileum derived from cows from the control (n = 5), shedder (n = 6), and clinical (n = 6) groups. Letters above columns indicate the result of the statistical analysis. Columns that do not share letters are significantly different (P < 0.05).
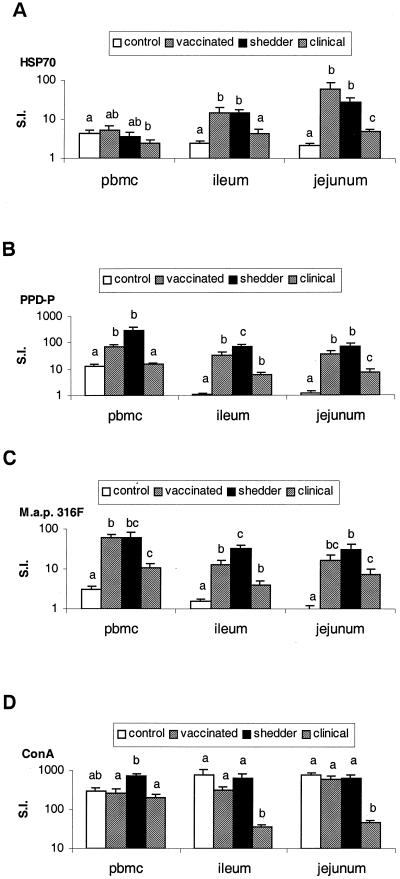
Lymphoproliferative responses of PBMC and MLMC. (i) Lymphoproliferative responses to Hsp70.
As depicted in Fig. 4A, the PBMC of control animals (mean SI = 4.3), vaccinated animals (mean SI = 5.4) and shedders (mean SI = 3.6) had comparable responses to Hsp70. Only the responses of animals with clinical Johne's disease (mean SI = 2.5) were lower than in control animals (P < 0.05). In the ileocecal MLMC, higher responses were found in vaccinated animals (mean SI = 15.0; P < 0.05) and shedders (mean SI = 14.3; P < 0.01) compared to control animals (mean SI = 2.4). Animals with clinical Johne's disease (mean SI = 4.4) had lower responses compared to shedders (P < 0.05) but greater responses compared to controls (P < 0.05). In the jejunal MLMC, all groups had greater responses to Hsp70 compared to controls (mean SI = 2.1) (P < 0.001). The vaccinated animals (mean SI = 60.5) and the shedders (mean SI = 27.5) had similar responses. The animals with clinical Johne's disease (mean SI = 4.9) had lower responses compared to vaccinated animals (P < 0.05) and shedders (P < 0.01) but still had higher responses than did the control animals (P < 0.001).
FIG. 4.
Lymphoproliferative responses of PBMC and MLNC. Proliferative responses of mononuclear lymphocyte populations isolated from blood (PBMC), the ileocecal lymph node (ileum), and a jejunal lymph node (jejunum). Responses to recombinant M. avium subsp. paratuberculosis Hsp70 (A), PPD-P (B), and whole bacteria (M. avium subsp. paratuberculosis strain 316F) (C) and the result of stimulation with the mitogen ConA (D) are illustrated. Responses, expressed as the SI (S.I.) plus the SE on a logarithmic scale, are depicted for animals from the control, vaccinated, shedder, and clinical groups. Different letters indicate statistically significant differences (P < 0.05) between the different groups, within an anatomical location.
Shedders had higher responses to Hsp70 in both ileocecal and jejunal MLMC compared to PBMC (both P < 0.05). Vaccinated animals also had higher responses to Hsp70 in ileocecal and jejunal MLMC (both P < 0.05). The magnitude of the responses was not related to the culture status of the vaccinated animals.
(ii) Lymphoproliferative responses to Hsp60.
Except for the positive responses in ileocecal MLMC of two of seven shedders, Hsp60 did not induce proliferative responses with an SI of 2 in the animals tested (data not shown).
(iii) Lymphoproliferative responses to PPD-P.
Compared to responses in control animals (mean SI = 12.9), higher responses were noted in vaccinated animals (mean SI = 71.8) (P < 0.001) and shedders (mean SI = 280.2) (P < 0.05), as shown in Fig. 4B. The vaccinated animals had PBMC responses to PPD-P that were comparable to those found in shedders. Animals with clinical Johne's disease (mean SI = 14.9) had lower responses compared to shedders (P < 0.05) and vaccinated animals (P < 0.001) but responses similar to those observed in control animals. Ileocecal and jejunal MLMC had similar response patterns except that animals with clinical Johne's disease (mean SI = 5.9 and 7.2, respectively) had higher responses compared to those observed in control animals (mean SI = 1.1 and 1.2, respectively; P < 0.001). The ileocecal and jejunal MLMC responses of control animals to PPD-P were much lower compared to the PBMC responses (P < 0.05 for both MLMC). Apart from the control group, no statistical significant differences were found between local and peripheral responses to PPD-P in the other groups.
(iv) Lymphoproliferative responses to M. avium subsp. paratuberculosis 316F.
As is shown in Fig. 4C, the PBMC of animals from the control group (mean SI = 3.0) had low proliferative responses to M. avium subsp. paratuberculosis 316F. The PBMC of vaccinated animals had higher responses compared to shedders (P < 0.05), although the difference is limited (mean SI = of 61.3 versus 59.1). Responses were decreased in animals with clinical Johne's disease (mean SI = 10.5) compared to shedders (P < 0.05) and vaccinated animals (P < 0.001) but still higher compared to those observed in control animals (P < 0.05). The patterns of response of ileocecal and jejunal MLMC were comparable to those observed for PBMC except that ileocecal MLMC of shedders (mean SI = 32.0 and 30.3, respectively) had higher responses compared to the MLMC of vaccinated animals (mean SI = 12.9 and 16.4, respectively; P < 0.05).
Only animals from the control group had lower responses in the ileocecal (mean SI = 1.5) and the jejunal (mean SI = 1.0) MLMC compared to the PBMC responses (P < 0.05). The other groups had local responses that are comparable to the responses observed in PBMC.
(v) Lymphoproliferative responses to ConA.
The mononuclear cell responses to the ConA mitogen are shown in Fig. 4D. The responses of PBMC to ConA are comparable between the control group, the vaccinated animals, and the animals with clinical disease. Mitogen-induced responses in the shedders were higher compared to vaccinated animals (P < 0.05) and animals with clinical signs of disease (P < 0.05) but similar to those of the control group. The ileocecal and jejunal MLMC of animals with clinical Johne's disease had lower responses compared to the other groups of animals and compared to their PBMC responses (all comparisons, P < 0.05). Only animals from the control group had higher responses in their ileocecal and jejunal MLMC compared to their PBMC (P < 0.05).
(vi) Lymphoproliferative responses of IEL and LPL.
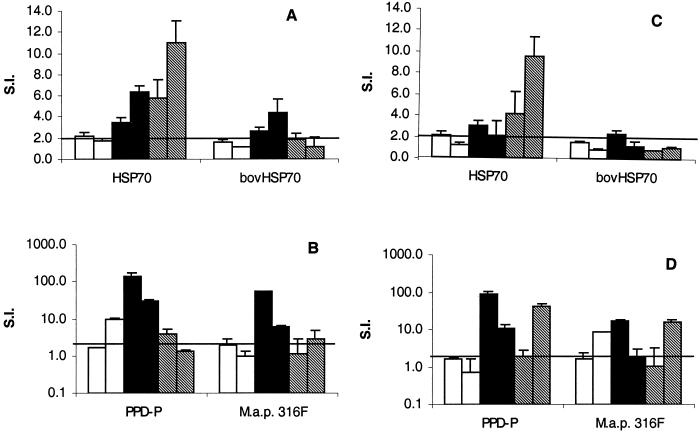
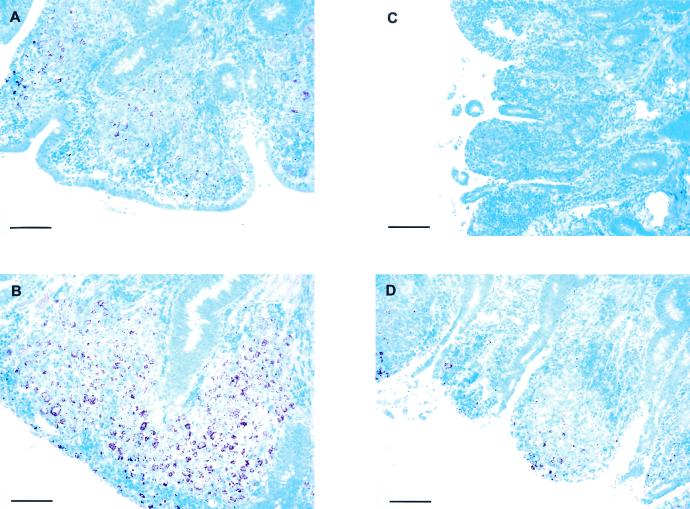
Flow cytometric analysis indicated that purity of the final preparations, with regard to the frequency of mononuclear cells versus contaminating epithelial cells, varied considerably in the LPL preparations (data not shown). Proliferative responses of ileum and jejunum LPL were found in cells derived from two clinical, two shedder, and two vaccinated animals (Fig. 5). The LPL cells from these six animals showed similar responses to ConA compared to other cell populations from the same animal. These cells also responded to PPD-P, Hsp70, and M. avium subsp. paratuberculosis whole-cell antigen. The shedders had the highest responses to these antigens compared to vaccinated animals and animals with clinical Johne's disease, except for responses to Hsp70, which were highest in the ileal LPL of animals with clinical Johne's disease. The responses of the vaccinated animals were comparable to the responses measured in animals with clinical Johne's disease. The animals with clinical disease had higher responses in their jejunal LPL compared to their ileal LPL, which was likely related to the level of infection, as judged by the histological appearance of the sections (Fig. 6).
FIG. 5.
Lymphoproliferative responses of LPL. Lymphoproliferative responses from vaccinated animals (□, n = 2), shedders (▪, n = 2), and animals with clinical signs (▧, n = 2) are expressed as the mean SI plus the SE from triplicate measurements. The responses of LPL isolated from the ileum and the jejunum to M. avium subsp. paratuberculosis Hsp70 and bovine Hsp70 are shown in panels A and C, respectively. The responses of LPL isolated from the ileum and the jejunum to M. avium subsp. paratuberculosis PPD-P and whole bacteria (M. avium subsp. paratuberculosis strain 316F [M.a.p. 316F]) are shown in panels B and D, respectively.
FIG. 6.
Photomicrographs of ileum and jejunum sections of cows with clinical signs of paratuberculosis. Photomicrographs of intestinal sections were stained according to Ziehl-Neelsen for detection of acid-fast bacteria. The panels show representative sections of the ileum (A and B) and the jejunum (C and D) of two cows with clinical signs of paratuberculosis. Panels A and C are from sections taken from the same animal; panels B and D are likewise from sections taken from the same animal. Bars, 100 μm.
Stimulation of IEL with mitogen or antigen did not result in proliferative responses, as measured by [3H]thymidine incorporation (data not shown).
DISCUSSION
To our knowledge, this is the first report to describe lymphocyte subset analysis and present a comparison of peripheral (PBMC) and local (MLNC and LPL) lymphoproliferation in different stages of bovine paratuberculosis induced by various M. avium subsp. paratuberculosis antigens.
The role of CD4+ Th1-type T cells and CD8+ T cells in protection against intracellular infections such as listeriosis, tuberculosis, and leprosy has been studied extensively (reviewed in reference 40). The role of γδ T cells in immunity to intracellular infections is still not fully understood, but they appear to contribute to protection in the early stages of infection (47). However, in later stages of disease, immunoregulatory γδ T cells may predominate, limiting inflammatory tissue damage (24). Based on functional and histopathological similarities with leprosy and tuberculosis, it may be argued that the various T-cell subsets play a similar role in bovine paratuberculosis (8, 14).
Several studies have shown previously in both cattle and sheep with multibacillary lesions that clinical Johne's disease is associated with a progressive loss of cell-mediated immune responses to mycobacterial antigens as measured by lymphoproliferation of PBMC and gamma interferon production ( (4, 9, 18, 28-30, 42). One of the possible explanations for this decline in peripheral responsiveness could be that, upon progression of the disease, M. avium subsp. paratuberculosis-specific T cells start homing preferentially to lesional sites due to increasing local antigenic load (33, 34, 36, 46) as the multiplication of bacteria increases. Our results showed that, whether or not redistribution of cells occurs, it does not result in increased proliferative responses in the MLMC of animals with clinical Johne's disease compared to shedders despite the increased antigenic stimulation, due to increased bacterial replication, in the former group.
Our results indicated that when the asymptomatic stage is compared with the clinical stage of Johne's disease, there is a decrease in the number of CD4+ T cells, whereas the CD8+ T cells remain at comparable frequencies. In addition, we found an increase of the γδ T-cell frequencies. These findings argue against effects caused by a space-occupying lesion (32), which would result in proportional changes in the subset frequencies. Our findings also indicate that there is a decrease in LPL proliferative responses to PPD-P, self-Hsp70, and whole bacteria in animals with clinical Johne's disease compared to asymptomatic shedders, indicating a loss of Th1-like activity. Together, these results support the hypothesis that in bovine paratuberculosis the pathogenesis may be mediated predominantly by the loss of protective CD4+-T-cell responses during the course of the disease (27, 43). The loss of CD4+ αβ and/or γδ T cells, through apoptotic cell death, has been described for experimental M. tuberculosis infection (17, 31, 35). Apoptotic deletion of T cells may, among others, be caused by γδ T cells (35) or increased TGF-β production (44). Some indications of increased transforming growth factor β1 (TGF-β1) expression have been found in paratuberculous sheep (2). In progressive bovine paratuberculosis, CD4+-T-cell-directed cytotoxic effects of γδ T cells may contribute to the course of the disease (11, 12). In addition, macrophages with increased numbers of apoptotic bodies have been noted in the lesional tissue of in cattle with clinical signs of disease (38), indicating an increased occurrence of apoptosis.
In contrast, the responses to mycobacterial Hsp70 do not tend to decrease in LPL of lesional tissue despite the significant decrease in the number of CD4+ cells. Recently, Kimura et al. (26) found M. tuberculosis Hsp70-reactive CD4+ T cells that produced TGF-β and interleukin-10 and downregulated Th1 T cells in a rat model for listeriosis, rendering the rats susceptible to infection. In murine listeriosis, γδ T cells have been described that apparently downregulate the Th1 responses that cause tissue damage during later stages of an inflammatory response (24, 37). Although hypothetical at this stage, in bovine paratuberculosis the remaining Hsp70 reactive T cells may be γδ T cells with a similar function, abrogating Th1-type immune responses.
In conclusion, we hypothesize that the loss of putatively protective CD4+ T cells leads to a lack of control of mycobacterial replication and, subsequently, to the progressive granulomatous enteritis typical of bovine paratuberculosis.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abbas, A., K. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Alzuherri, H. M., C. J. Woodall, and C. J. Clarke. 1996. Increased intestinal TNF-α, IL-1β and IL-6 expression in ovine paratuberculosis. Vet. Immunol. Immunopathol. 49:331-345. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin, C. L., A. J. Teale, J. G. Naessens, B. M. Goddeeris, N. D. MacHugh, and W. I. Morrison. 1986. Characterization of a subset of bovine T lymphocytes that express BoT4 by monoclonal antibodies and function: similarity to lymphocytes defined by human T4 and murine L3T4. J. Immunol. 136:4385-4391. [PubMed] [Google Scholar]
- 4.Bendixen, P. H. 1978. Immunological reactions caused by infection with Mycobacterium paratuberculosis: a review. Nord. Vet. Med. 30:163-168. [PubMed] [Google Scholar]
- 5.Bloom, B. R., P. Salgame, and B. Diamond. 1992. Revisiting and revising suppressor T cells. Immunol. Today 13:131-136. [DOI] [PubMed] [Google Scholar]
- 6.Brodersen, R., F. Bijlsma, K. Gori, K. T. Jensen, W. Chen, J. Dominguez, K. Haverson, P. F. Moore, A. Saalmuller, D. Sachs, W. J. Slierendrecht, C. Stokes, O. Vainio, F. Zuckermann, and B. Aasted. 1998. Analysis of the immunological cross reactivities of 213-well characterized monoclonal antibodies with specificities against various leucocyte surface antigens of human and 11 animal species. Vet. Immunol. Immunopathol. 64:1-13. [DOI] [PubMed] [Google Scholar]
- 7.Brown, W. C., A. C. Rice-Ficht, and D. M. Estes. 1998. Bovine type 1 and type 2 responses. Vet. Immunol. Immunopathol. 63:45-55. [DOI] [PubMed] [Google Scholar]
- 8.Buergelt, C. D., C. Hall, K. McEntee, and J. R. Duncan. 1978. Pathological evaluation of paratuberculosis in naturally infected cattle. Vet. Pathol. 15:196-207. [DOI] [PubMed] [Google Scholar]
- 9.Burrells, C., C. J. Clarke, A. Colston, J. M. Kay, J. Porter, D. Little, and J. M. Sharp. 1998. A study of immunological responses of sheep clinically affected with paratuberculosis (Johne's disease) The relationship of blood, mesenteric lymph node and intestinal lymphocyte responses to gross and microscopic pathology. Vet. Immunol. Immunopathol. 66:343-358. [DOI] [PubMed] [Google Scholar]
- 10.Chiodini, R. J. 1996. Immunology: resistance to paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 12:313-343. [DOI] [PubMed] [Google Scholar]
- 11.Chiodini, R. J., and W. C. Davis. 1993. The cellular immunology of bovine paratuberculosis: immunity may be regulated by CD4+ helper and CD8+ immunoregulatory T lymphocytes which downregulate γ/δ+ T-cell cytotoxicity. Microb. Pathog. 14:355-367. [DOI] [PubMed] [Google Scholar]
- 12.Chiodini, R. J., and W. C. Davis. 1992. The cellular immunology of bovine paratuberculosis: the predominant response is mediated by cytotoxic γ/δ T lymphocytes which prevent CD4+ activity. Microb. Pathog. 13:447-463. [DOI] [PubMed] [Google Scholar]
- 13.Chiodini, R. J., H. J. Van Kruiningen, and R. S. Merkal. 1984. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 74:218-262. [PubMed] [Google Scholar]
- 14.Clarke, C. J. 1997. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J. Comp. Pathol. 116:217-261. [DOI] [PubMed] [Google Scholar]
- 15.Clevers, H., N. D. MacHugh, A. Bensaid, S. Dunlap, C. L. Baldwin, A. Kaushal, K. Iams, C. J. Howard, and W. I. Morrison. 1990. Identification of a bovine surface antigen uniquely expressed on CD4− CD8− T-cell receptor γ/δ+ T lymphocytes. Eur. J. Immunol. 20:809-817. [DOI] [PubMed] [Google Scholar]
- 16.Colston, A., I. McConnell, and R. Bujdoso. 1994. Cloning and expression in Escherichia coli of DNA encoding a 60-kDa stress protein of Mycobacterium paratuberculosis, the causative agent of Johne's disease. Microbiology 140:3329-3336. [DOI] [PubMed] [Google Scholar]
- 17.Das, G., H. Vohra, B. Saha, J. N. Agrewala, and G. C. Mishra. 1999. Apoptosis of Th1-like cells in experimental tuberculosis (TB). Clin. Exp. Immunol. 115:324-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies, D. H., L. Corbeil, D. Ward, and J. R. Duncan. 1974. A humoral suppressor of in vitro lymphocyte transformation responses in cattle with Johne's disease. Proc. Soc. Exp. Biol. Med. 145:1372-1377. [DOI] [PubMed] [Google Scholar]
- 19.Davis, W. C., W. C. Brown, M. J. Hamilton, C. R. Wyatt, J. A. Orden, A. M. Khalid, and J. Naessens. 1996. Analysis of monoclonal antibodies specific for the gamma delta TcR. Vet. Immunol. Immunopathol. 52:275-283. [DOI] [PubMed] [Google Scholar]
- 20.Davis, W. C., J. A. Ellis, N. D. MacHugh, and C. L. Baldwin. 1988. Bovine pan T-cell monoclonal antibodies reactive with a molecule similar to CD2. Immunology 63:165-167. [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis, J. A., C. L. Baldwin, N. D. MacHugh, A. Bensaid, A. J. Teale, B. M. Goddeeris, and W. I. Morrison. 1986. Characterization by a monoclonal antibody and functional analysis of a subset of bovine T lymphocytes that express BoT8, a molecule analogous to human CD8. Immunology 58:351-358. [PMC free article] [PubMed] [Google Scholar]
- 22.Gilmour, N. J. L., and G. W. Wood. 1996. Paratuberculosis (Johne's disease), p. 218-228. In OIE manual of standards for diagnostic tests and vaccines, 3rd ed. Office International des Epizooties, Paris, France.
- 23.Howard, C. J., W. I. Morrison, A. Bensaid, W. Davis, L. Eskra, J. Gerdes, M. Hadam, D. Hurley, W. Leibold, J. J. Letesson, et al. 1991. Summary of workshop findings for leukocyte antigens of cattle. Vet. Immunol. Immunopathol. 27:21-27. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh, B., M. D. Schrenzel, T. Mulvania, H. D. Lepper, L. DiMolfetto-Landon, and D. A. Ferrick. 1996. In vivo cytokine production in murine listeriosis: evidence for immunoregulation by γδ+ T cells. J. Immunol. 156:232-237. [PubMed] [Google Scholar]
- 25.Jorgensen, J. B. 1982. An improved medium for culture of Mycobacterium paratuberculosis from bovine faeces. Acta Vet. Scand. 23:325-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura, Y., K. Yamada, T. Sakai, K. Mishima, H. Nishimura, Y. Matsumoto, M. Singh, and Y. Yoshikai. 1998. The regulatory role of heat shock protein 70-reactive CD4+ T cells during rat listeriosis. Int. Immunol. 10:117-130. [DOI] [PubMed] [Google Scholar]
- 27.Koets, A. P., V. P. Rutten, M. de Boer, D. Bakker, P. Valentin-Weigand, and W. van Eden. 2001. Differential changes in heat shock protein-, lipoarabinomannan-, and purified protein derivative-specific immunoglobulin G1 and G2 isotype responses during bovine Mycobacterium avium subsp. paratuberculosis infection. Infect. Immun. 69:1492-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koets, A. P., V. P. Rutten, A. Hoek, D. Bakker, F. van Zijderveld, K. E. Muller, and W. van Eden. 1999. Heat-shock protein-specific T-cell responses in various stages of bovine paratuberculosis. Vet. Immunol. Immunopathol. 70:105-115. [DOI] [PubMed] [Google Scholar]
- 29.Kreeger, J. M., and T. G. den Snider. 1992. Measurement of lymphoblast proliferative capacity of stimulated blood mononuclear cells from cattle with chronic paratuberculosis. Am. J. Vet. Res. 53:392-395. [PubMed] [Google Scholar]
- 30.Kreeger, J. M., T. G. den Snider, and B. M. Olcott. 1992. Effects of dialyzable lymph node extracts on lymphoblast proliferative capacity of blood mononuclear cells in cattle with chronic paratuberculosis. Am. J. Vet. Res. 53:1225-1230. [PubMed] [Google Scholar]
- 31.Li, B., H. Bassiri, M. D. Rossman, P. Kramer, A. F. Eyuboglu, M. Torres, E. Sada, T. Imir, and S. R. Carding. 1998. Involvement of the Fas/Fas ligand pathway in activation-induced cell death of mycobacterium-reactive human γδ T cells: a mechanism for the loss of γδ T cells in patients with pulmonary tuberculosis. J. Immunol. 161:1558-1567. [PubMed] [Google Scholar]
- 32.Little, D., H. M. Alzuherri, and C. J. Clarke. 1996. Phenotypic characterisation of intestinal lymphocytes in ovine paratuberculosis by immunohistochemistry. Vet. Immunol. Immunopathol. 55:175-187. [DOI] [PubMed] [Google Scholar]
- 33.Mackay, C. R. 1993. Homing of naive, memory and effector lymphocytes. Curr. Opin. Immunol. 5:423-427. [DOI] [PubMed] [Google Scholar]
- 34.Mackay, C. R. 1992. Migration pathways and immunologic memory among T lymphocytes. Semin. Immunol. 4:51-58. [PubMed] [Google Scholar]
- 35.Manfredi, A. A., S. Heltai, P. Rovere, C. Sciorati, C. Paolucci, G. Galati, C. Rugarli, R. Vaiani, E. Clementi, and M. Ferrarini. 1998. Mycobacterium tuberculosis exploits the CD95/CD95 ligand system of γδ T cells to cause apoptosis. Eur. J. Immunol. 28:1798-1806. [DOI] [PubMed] [Google Scholar]
- 36.Meeusen, E. N. 1998. Differential migration of Th1 and Th2 cells: implications for vaccine and infection studies. Vet. Immunol. Immunopathol. 63:157-166. [DOI] [PubMed] [Google Scholar]
- 37.Mombaerts, P., J. Arnoldi, F. Russ, S. Tonegawa, and S. H. Kaufmann. 1993. Different roles of αβ and γδ T cells in immunity against an intracellular bacterial pathogen. Nature 365:53-56. [DOI] [PubMed] [Google Scholar]
- 38.Momotani, E., M. Yamaguchi, K. Tanaka, Y. Arita, M. Iga, and K. Yoshihara. 1999. Shedding mechanism of M. avium subspecies paratuberculosis from intestinal mucosa, p. 675. In 6th International Colloquium on Paratuberculosis. IAP, Inc., Madison, Wis.
- 39.Mosmann, T. R., H. Cherwinski, M. W. Bond, M. A. Giedlin, and R. L. Coffman. 1986. Two types of murine helper T-cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348-2357. [PubMed] [Google Scholar]
- 40.Schaible, U. E., H. L. Collins, and S. H. E. Kaufmann. 1999. Confrontation between intracellular bacteria and the immune system. Adv. Immunol. 71:267-377. [DOI] [PubMed] [Google Scholar]
- 41.Siegel, S., and N. J. Castellan, Jr. 1988. Nonparametric statistics for the behavioral sciences, 2nd ed. McGraw-Hill, Singapore.
- 42.Stabel, J. R. 1996. Production of gamma-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of subclinical paratuberculosis. J. Vet. Diagn. Investig. 8:345-350. [DOI] [PubMed] [Google Scholar]
- 43.Sweeney, R., D. Jones, R. Whitlock, and P. Scott. 1996. Cytokine expression in intestinal tissue of cows infected with Mycobacterium paratuberculosis, p. 48-51. In Fifth International Colloquium on Paratuberculosis. Evergreen Press, East Providence, R.I.
- 44.Van Parijs, L., and A. K. Abbas. 1998. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science 280:243-248. [DOI] [PubMed] [Google Scholar]
- 45.Vary, P. H., P. R. Andersen, E. Green, J. Hermon-Taylor, and J. J. McFadden. 1990. Use of highly specific DNA probes and the polymerase chain reaction to detect Mycobacterium paratuberculosis in Johne's disease. J. Clin. Microbiol. 28:933-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Washington, E. A., M. Katerelos, R. N. Cahill, and W. G. Kimpton. 1994. Differences in the tissue-specific homing of αβ and γδ T cells to gut and peripheral lymph nodes. Int. Immunol. 6:1891-1897. [DOI] [PubMed] [Google Scholar]
- 47.Ziegler, H., M. Skeen, and K. Pearce. 1994. Role of α/β T and γ/δ T cells in innate and acquired immunity. Ann. N. Y. Acad. Sci. 730:53-70. [DOI] [PubMed] [Google Scholar]