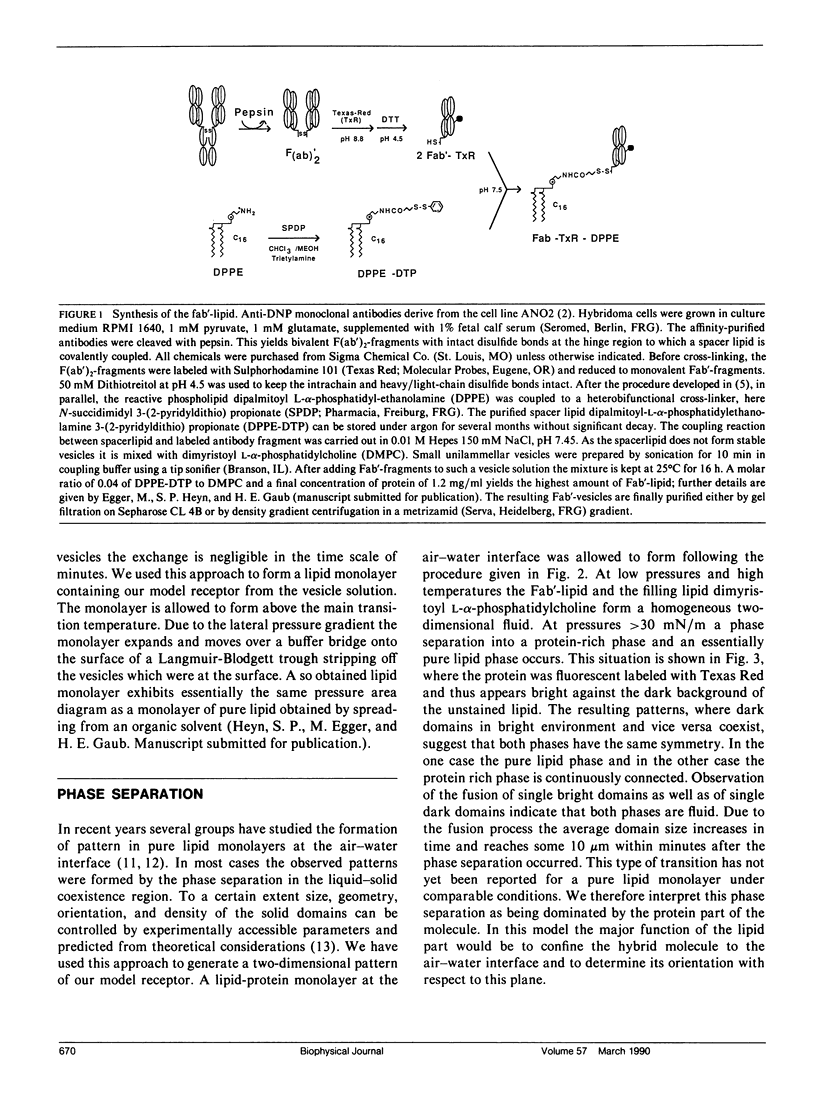
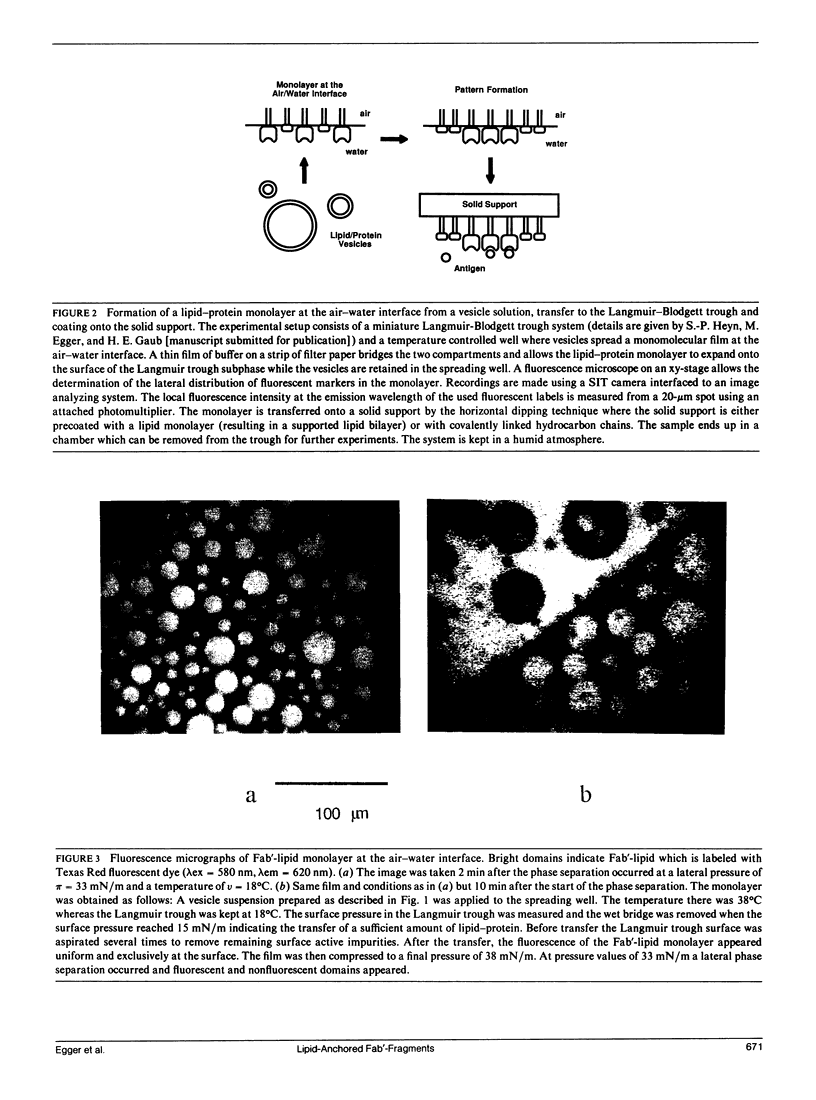
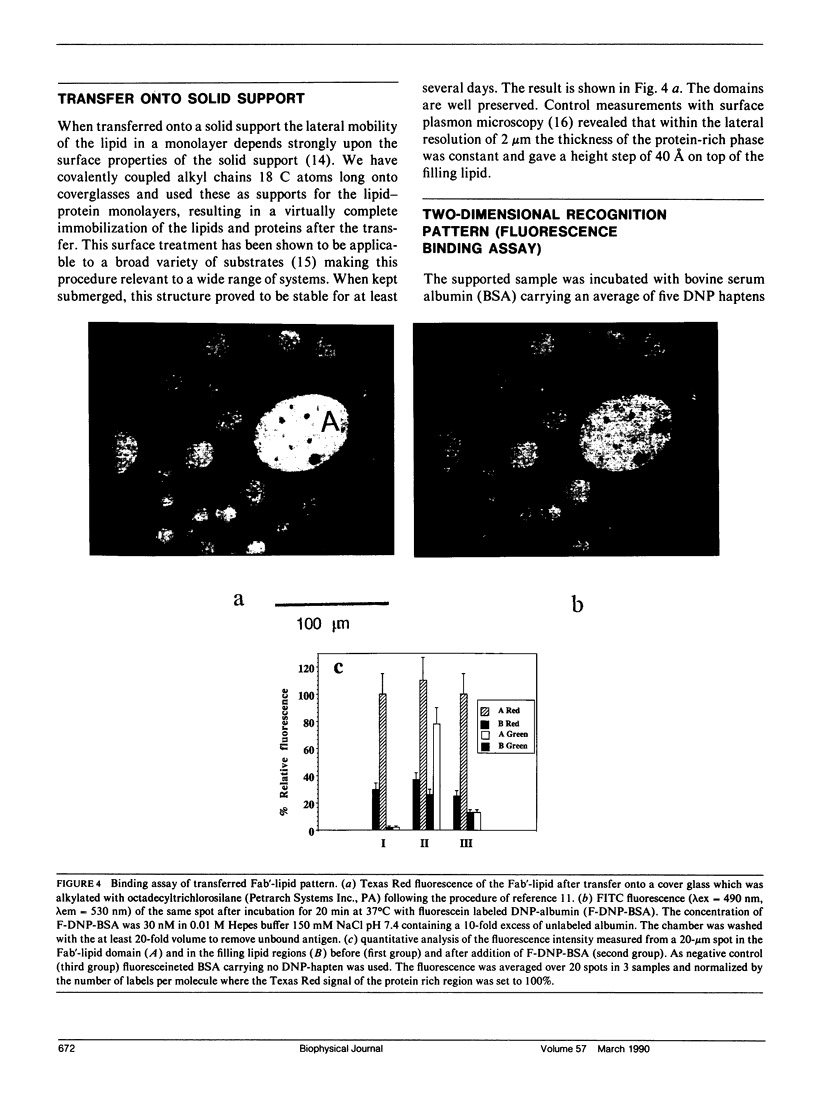
Abstract
A two-dimensional pattern of oriented antibody fragments was formed at the air-water interface and transferred onto a solid support. The Fab'-fragments of a monoclonal antibody against the hapten dinitrophenyl (DNP) were covalently linked via a hydrophilic spacer to phospholipid vesicles. A monomolecular lipid-protein layer at equilibrium with these vesicles was allowed to form at the air-water interface. The monolayer was separated from the vesicle phase and transferred to a Langmuir-Blodgett trough. By cooling and compressing, the previously homogeneous lipid-protein film was driven into a two-dimensional phase separation resulting in protein-rich domains and a second phase consisting mainly of lipid. This film was transferred onto a solid support in a way that preserved the protein-lipid pattern. The specificity as well as the contrast in the binding activity of the two different separated phases were then quantified using microfluorometry. DNP conjugated to fluorescein-labeled bovine serum albumin (BSA) showed virtually no binding to the lipid regions, but gave a ratio of bound DNP-BSA to Fab'-lipid of greater than 50% in the protein-rich domains proving that the Fab'-moiety retained its biological activity. This demonstrates that the technique presented here is well suited to modify different solid surfaces with a pattern of a given biological function. The optional control of lateral packing and orientation of the components in the monolayer makes it a general tool for the reconstitution of supported lipid-protein membranes and might also open new ways for the two-dimensional crystallization of proteins at membranes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balakrishnan K., Hsu F. J., Hafeman D. G., McConnell H. M. Monoclonal antibodies to a nitroxide lipid hapten. Biochim Biophys Acta. 1982 Sep 13;721(1):30–38. doi: 10.1016/0167-4889(82)90020-9. [DOI] [PubMed] [Google Scholar]
- Blankenburg R., Meller P., Ringsdorf H., Salesse C. Interaction between biotin lipids and streptavidin in monolayers: formation of oriented two-dimensional protein domains induced by surface recognition. Biochemistry. 1989 Oct 3;28(20):8214–8221. doi: 10.1021/bi00446a037. [DOI] [PubMed] [Google Scholar]
- Brian A. A., McConnell H. M. Allogeneic stimulation of cytotoxic T cells by supported planar membranes. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6159–6163. doi: 10.1073/pnas.81.19.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Martin F. J., Hubbell W. L., Papahadjopoulos D. Immunospecific targeting of liposomes to cells: a novel and efficient method for covalent attachment of Fab' fragments via disulfide bonds. Biochemistry. 1981 Jul 7;20(14):4229–4238. doi: 10.1021/bi00517a043. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., Watts T. H., Weis R. M., Brian A. A. Supported planar membranes in studies of cell-cell recognition in the immune system. Biochim Biophys Acta. 1986 Jun 12;864(1):95–106. doi: 10.1016/0304-4157(86)90016-x. [DOI] [PubMed] [Google Scholar]
- Schindler H. Exchange and interactions between lipid layers at the surface of a liposome solution. Biochim Biophys Acta. 1979 Aug 7;555(2):316–336. doi: 10.1016/0005-2736(79)90171-8. [DOI] [PubMed] [Google Scholar]
- Verger R., Pattus F. Spreading of membranes at the air/water interface. Chem Phys Lipids. 1976 Jul;16(4):285–291. doi: 10.1016/0009-3084(76)90023-2. [DOI] [PubMed] [Google Scholar]