Abstract
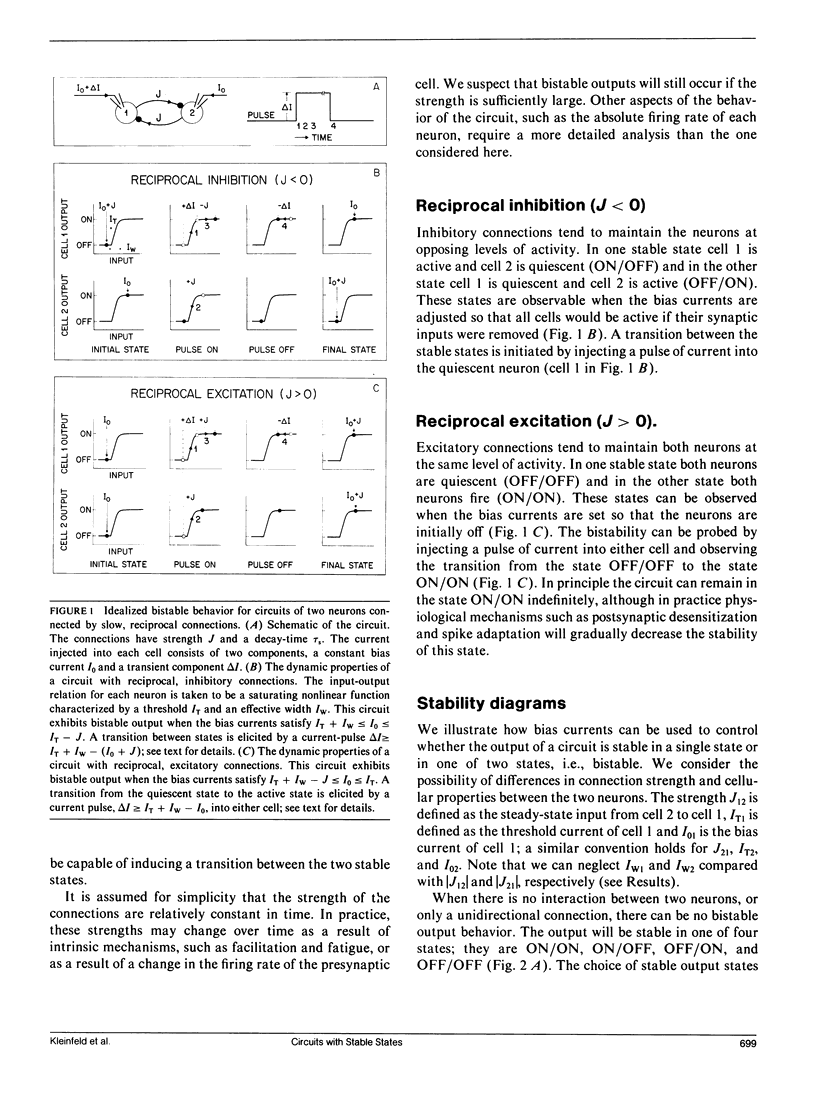
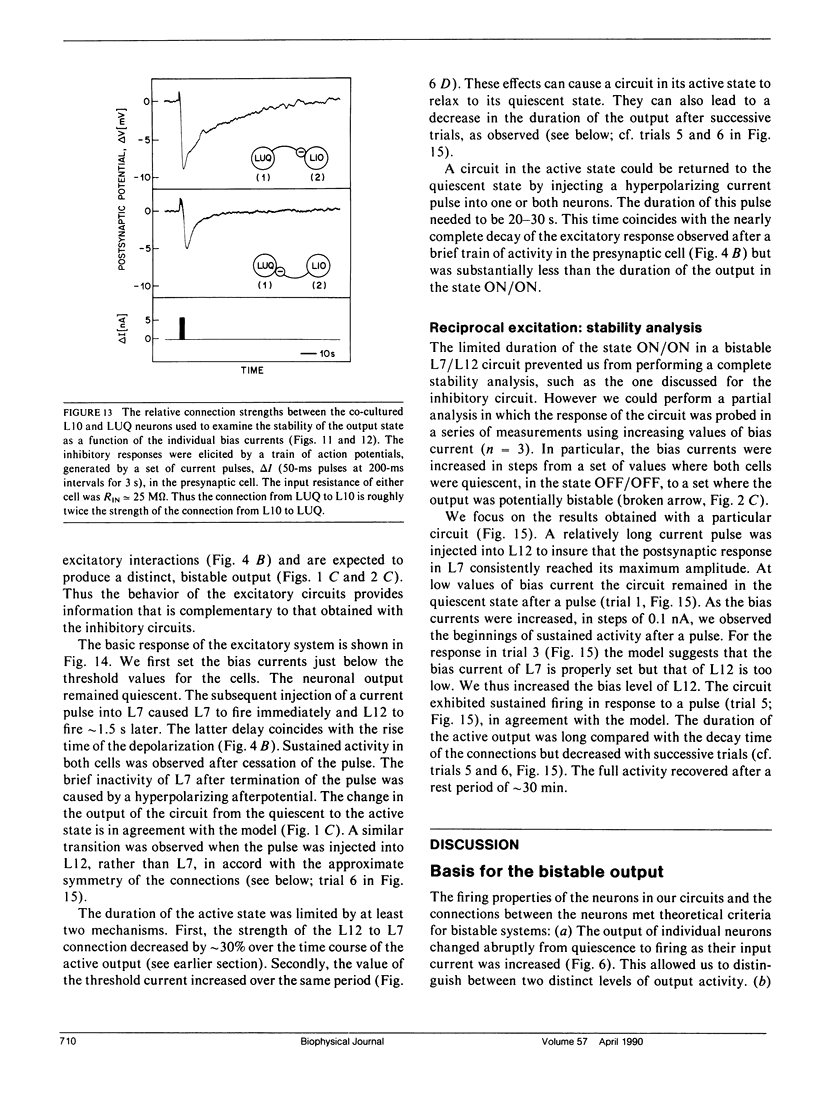
We have used identified neurons from the abdominal ganglion of the mollusc Aplysia to construct and analyze two circuits in vitro. Each of these circuits was capable of producing two patterns of persistent activity; that is, they had bistable output states. The output could be switched between the stable states by a brief, external input. One circuit consisted of cocultured L10 and left upper quadrant (LUQ) neurons that formed reciprocal, inhibitory connections. In one stable state L10 was active and the LUQ was quiescent, whereas in the other stable state L10 was quiescent and the LUQ was active. A second circuit consisted of co-cultured L7 and L12 neurons that formed reciprocal, excitatory connections. In this circuit, both cells were quiescent in one stable state and both cells fired continuously in the other state. Bistable output in both circuits resulted from the nonlinear firing characteristics of each neuron and the feedback between the two neurons. We explored how the stability of the neuronal output could be controlled by the background currents injected into each neuron. We observed a relatively well-defined range of currents for which bistability occurred, consistent with the values expected from the measured strengths of the connections and a simple model. Outside of the range, the output was stable in only a single state. These results suggest how stable patterns of output are produced by some in vivo circuits and how command neurons from higher neural centers may control the activity of these circuits. The criteria that guided us in forming our circuits in culture were derived from theoretical studies on the properties of certain neuronal network models (e.g., Hopfield, J. J. 1984. Proc. Natl. Acad. Sci. USA. 81:3088-3092). Our results show that circuits consisting of only two co-cultured neurons can exhibit bistable output states of the form hypothesized to occur in populations of neurons.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amit DJ, Gutfreund H, Sompolinsky H. Spin-glass models of neural networks. Phys Rev A Gen Phys. 1985 Aug;32(2):1007–1018. doi: 10.1103/physreva.32.1007. [DOI] [PubMed] [Google Scholar]
- Amit DJ, Gutfreund H, Sompolinsky H. Storing infinite numbers of patterns in a spin-glass model of neural networks. Phys Rev Lett. 1985 Sep 30;55(14):1530–1533. doi: 10.1103/PhysRevLett.55.1530. [DOI] [PubMed] [Google Scholar]
- Dagan D., Levitan I. B. Isolated identified Aplysia neurons in cell culture. J Neurosci. 1981 Jul;1(7):736–740. doi: 10.1523/JNEUROSCI.01-07-00736.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S., Changeux J. P., Nadal J. P. Neural networks that learn temporal sequences by selection. Proc Natl Acad Sci U S A. 1987 May;84(9):2727–2731. doi: 10.1073/pnas.84.9.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcomyn F. Neural basis of rhythmic behavior in animals. Science. 1980 Oct 31;210(4469):492–498. doi: 10.1126/science.7423199. [DOI] [PubMed] [Google Scholar]
- Fuster J. M., Jervey J. P. Neuronal firing in the inferotemporal cortex of the monkey in a visual memory task. J Neurosci. 1982 Mar;2(3):361–375. doi: 10.1523/JNEUROSCI.02-03-00361.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getting P. A., Dekin M. S. Mechanisms of pattern generation underlying swimming in Tritonia. IV. Gating of central pattern generator. J Neurophysiol. 1985 Feb;53(2):466–480. doi: 10.1152/jn.1985.53.2.466. [DOI] [PubMed] [Google Scholar]
- Getting P. A. Mechanisms of pattern generation underlying swimming in Tritonia. I. Neuronal network formed by monosynaptic connections. J Neurophysiol. 1981 Jul;46(1):65–79. doi: 10.1152/jn.1981.46.1.65. [DOI] [PubMed] [Google Scholar]
- Getting P. A. Mechanisms of pattern generation underlying swimming in Tritonia. III. Intrinsic and synaptic mechanisms for delayed excitation. J Neurophysiol. 1983 Apr;49(4):1036–1050. doi: 10.1152/jn.1983.49.4.1036. [DOI] [PubMed] [Google Scholar]
- Harth E. M., Csermely T. J., Beek B., Lindsay R. D. Brain functions and neural dynamics. J Theor Biol. 1970 Jan;26(1):93–120. doi: 10.1016/s0022-5193(70)80035-2. [DOI] [PubMed] [Google Scholar]
- Hopfield J. J. Neural networks and physical systems with emergent collective computational abilities. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield J. J. Neurons with graded response have collective computational properties like those of two-state neurons. Proc Natl Acad Sci U S A. 1984 May;81(10):3088–3092. doi: 10.1073/pnas.81.10.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek L. K., Finbow M., Revel J. P., Strumwasser F. The morphology and coupling of Aplysia bag cells within the abdominal ganglion and in cell culture. J Neurobiol. 1979 Nov;10(6):535–550. doi: 10.1002/neu.480100604. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Frazier W. T., Waziri R., Coggeshall R. E. Direct and common connections among identified neurons in Aplysia. J Neurophysiol. 1967 Nov;30(6):1352–1376. doi: 10.1152/jn.1967.30.6.1352. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D. Sequential state generation by model neural networks. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9469–9473. doi: 10.1073/pnas.83.24.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D., Sompolinsky H. Associative neural network model for the generation of temporal patterns. Theory and application to central pattern generators. Biophys J. 1988 Dec;54(6):1039–1051. doi: 10.1016/S0006-3495(88)83041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester J., Alevizos A. Innervation of the kidney of Aplysia by L10, the LUQ cells, and an identified peripheral motoneuron. J Neurosci. 1989 Nov;9(11):4078–4088. doi: 10.1523/JNEUROSCI.09-11-04078.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester J., Kandel E. R. Further identification of neurons in the abdominal ganglion of Aplysia using behavioral criteria. Brain Res. 1977 Jan 31;121(1):1–20. doi: 10.1016/0006-8993(77)90435-8. [DOI] [PubMed] [Google Scholar]
- Koester J., Mayeri E., Liebeswar G., Kandel E. R. Neural control of circulation in Aplysia. II. Interneurons. J Neurophysiol. 1974 May;37(3):476–496. doi: 10.1152/jn.1974.37.3.476. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Zucker R. S. Calcium-induced inactivation of calcium current causes the inter-burst hyperpolarization of Aplysia bursting neurones. J Physiol. 1985 May;362:131–160. doi: 10.1113/jphysiol.1985.sp015667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Miller J. P., Selverston A. I. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. IV. Network properties of pyloric system. J Neurophysiol. 1982 Dec;48(6):1416–1432. doi: 10.1152/jn.1982.48.6.1416. [DOI] [PubMed] [Google Scholar]
- Nagy F., Dickinson P. S. Control of a central pattern generator by an identified modulatory interneurone in crustacea. I. Modulation of the pyloric motor output. J Exp Biol. 1983 Jul;105:33–58. doi: 10.1242/jeb.105.1.33. [DOI] [PubMed] [Google Scholar]
- Peterson E. L., Calabrese R. L. Dynamic analysis of a rhythmic neural circuit in the leech Hirudo medicinalis. J Neurophysiol. 1982 Feb;47(2):256–271. doi: 10.1152/jn.1982.47.2.256. [DOI] [PubMed] [Google Scholar]
- Schacher S., Proshansky E. Neurite regeneration by Aplysia neurons in dissociated cell culture: modulation by Aplysia hemolymph and the presence of the initial axonal segment. J Neurosci. 1983 Dec;3(12):2403–2413. doi: 10.1523/JNEUROSCI.03-12-02403.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz K. P., Cleary L. J., Byrne J. H. Inositol 1,4,5-trisphosphate alters bursting pacemaker activity in Aplysia neurons: voltage-clamp analysis of effects on calcium currents. J Neurophysiol. 1988 Jul;60(1):86–104. doi: 10.1152/jn.1988.60.1.86. [DOI] [PubMed] [Google Scholar]
- Sompolinsky H, Kanter I., I Temporal association in asymmetric neural networks. Phys Rev Lett. 1986 Dec 1;57(22):2861–2864. doi: 10.1103/PhysRevLett.57.2861. [DOI] [PubMed] [Google Scholar]
- Wilson H. R., Cowan J. D. Excitatory and inhibitory interactions in localized populations of model neurons. Biophys J. 1972 Jan;12(1):1–24. doi: 10.1016/S0006-3495(72)86068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]