Abstract
Using the outside-out patch clamp recording technique together with a rapid solution exchange system, we measured ionic currents through nicotinic acetylcholine (ACh) receptor channels from BC3H-1 cells in response to rapid applications of 0.3-1,000 microM ACh. We used nonstationary fluctuation analysis of ensembles of responses to deduce the number of channels in the patch, the maximum open channel probability as a function of ACh concentration and the time course of a fast desensitization process. We found that: (a) Excised patches from BC3H-1 cells typically contain between 50 and 150 functional ACh receptor ion channels. (b) The open channel probability is proportional to [ACh]1.95 at low concentrations of ACh, is half-maximal at 20 microM ACh and saturates above 100 microM ACh. (c) ACh is a very efficacious agonist; 100 microM ACh opens at least 90% of the available channels. This estimate of efficacy is model-independent. (d) The rate of decay of the agonist-induced current is concentration-dependent. In the presence of 100 microM ACh the current decays with a time constant of 50-100 ms. It decays more slowly in the presence of lower concentrations of agonist but is relatively insensitive to voltage.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. An analysis of the dose-response curve at voltage-clamped frog-endplates. Pflugers Arch. 1975 Oct 28;360(2):145–153. doi: 10.1007/BF00580537. [DOI] [PubMed] [Google Scholar]
- Adams P. R., Feltz A. End-plate channel opening and the kinetics of quinacrine (mepacrine) block. J Physiol. 1980 Sep;306:283–306. doi: 10.1113/jphysiol.1980.sp013397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R., Narahashi T. Desensitization of the acetylcholine receptor of denervated rat soleus muscle and the effect of calcium. Br J Pharmacol. 1980 May;69(1):91–98. doi: 10.1111/j.1476-5381.1980.tb10886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A., Lingle C. J. Heterogeneous kinetic properties of acetylcholine receptor channels in Xenopus myocytes. J Physiol. 1986 Sep;378:119–140. doi: 10.1113/jphysiol.1986.sp016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J., Patrick J. Purification of an acetylcholine receptor from a nonfusing muscle cell line. Biochemistry. 1977 Nov 1;16(22):4900–4908. doi: 10.1021/bi00641a025. [DOI] [PubMed] [Google Scholar]
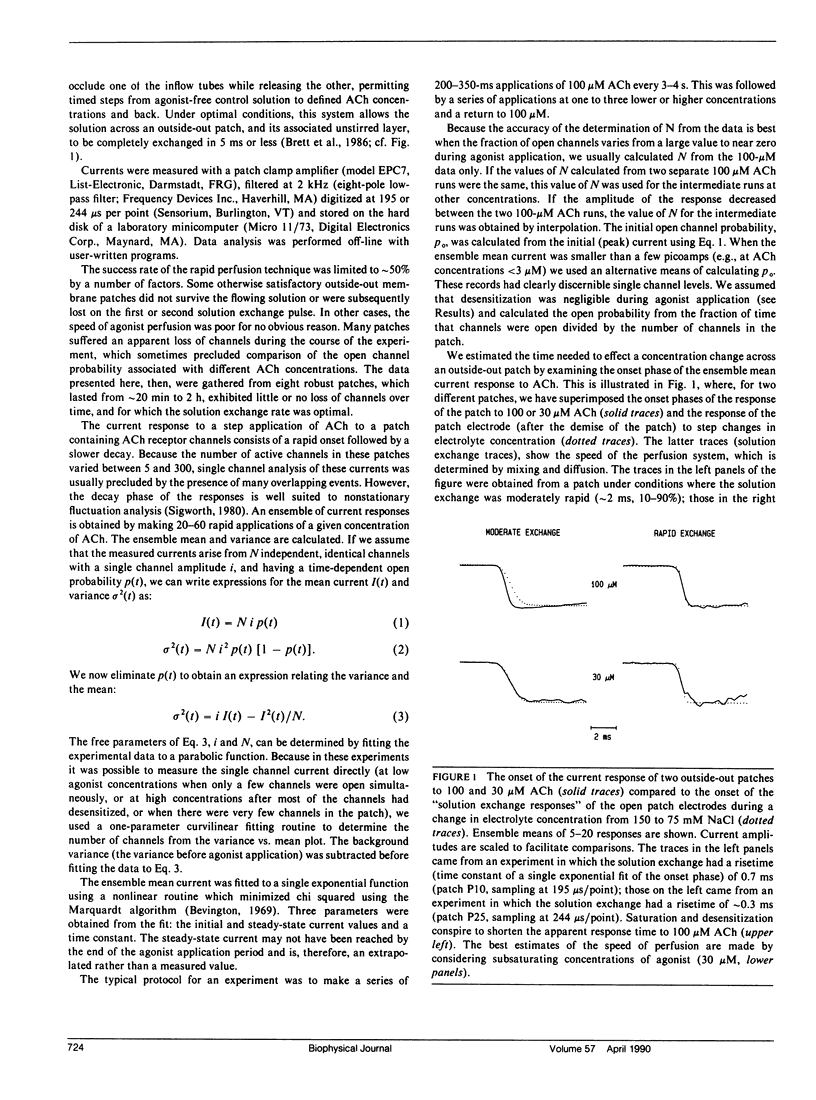
- Brett R. S., Dilger J. P., Adams P. R., Lancaster B. A method for the rapid exchange of solutions bathing excised membrane patches. Biophys J. 1986 Nov;50(5):987–992. doi: 10.1016/S0006-3495(86)83539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Sodium transport by the acetylcholine receptor of cultured muscle cells. J Biol Chem. 1975 Mar 10;250(5):1776–1781. [PubMed] [Google Scholar]
- Chabala L. D., Gurney A. M., Lester H. A. Dose-response of acetylcholine receptor channels opened by a flash-activated agonist in voltage-clamped rat myoballs. J Physiol. 1986 Feb;371:407–433. doi: 10.1113/jphysiol.1986.sp015983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabala L. D., Gurney A. M., Lester H. A. Photoactivation and dissociation of agonist molecules at the nicotinic acetylcholine receptor in voltage-clamped rat myoballs. Biophys J. 1985 Aug;48(2):241–246. doi: 10.1016/S0006-3495(85)83777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut T. J. Two-component desensitization at the neuromuscular junction of the frog. J Physiol. 1983 Mar;336:229–241. doi: 10.1113/jphysiol.1983.sp014578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Ogden D. C. Activation of ion channels in the frog end-plate by high concentrations of acetylcholine. J Physiol. 1988 Jan;395:131–159. doi: 10.1113/jphysiol.1988.sp016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Steinbach J. H., Stevens C. F. An analysis of the dose-response relationship at voltage-clamped frog neuromuscular junctions. J Physiol. 1978 Aug;281:421–444. doi: 10.1113/jphysiol.1978.sp012431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K., Sterz R. Determination of dose-response curves by quantitative ionophoresis at the frog neuromuscular junction. J Physiol. 1978 Aug;281:395–419. doi: 10.1113/jphysiol.1978.sp012430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman S. A., Miller K. W. High acetylcholine concentrations cause rapid inactivation before fast desensitization in nicotinic acetylcholine receptors from Torpedo. Biophys J. 1988 Jul;54(1):149–158. doi: 10.1016/S0006-3495(88)82939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Korenbrot J. I., Maricq A. V. Kinetics of activation of acetylcholine receptors in a mouse muscle cell line under a range of acetylcholine concentrations. Biophys J. 1987 Mar;51(3):449–455. doi: 10.1016/S0006-3495(87)83366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Dependence of acetylcholine receptor channel kinetics on agonist concentration in cultured mouse muscle fibres. J Physiol. 1988 Mar;397:555–583. doi: 10.1113/jphysiol.1988.sp017019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A., Changeux J. P., Sheridan R. E. Conductance increases produced by bath application of cholinergic agonists to Electrophorus electroplaques. J Gen Physiol. 1975 Jun;65(6):797–816. doi: 10.1085/jgp.65.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. A study of desensitization of acetylcholine receptors using nerve-released transmitter in the frog. J Physiol. 1981 Jul;316:225–250. doi: 10.1113/jphysiol.1981.sp013784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Ogden D. C., Colquhoun D. Ion channel block by acetylcholine, carbachol and suberyldicholine at the frog neuromuscular junction. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):329–355. doi: 10.1098/rspb.1985.0065. [DOI] [PubMed] [Google Scholar]
- Ogden D. C., Colquhoun D. The efficacy of agonists at the frog neuromuscular junction studied with single channel recording. Pflugers Arch. 1983 Nov;399(3):246–248. doi: 10.1007/BF00656725. [DOI] [PubMed] [Google Scholar]
- Papke R. L., Millhauser G., Lieberman Z., Oswald R. E. Relationships of agonist properties to the single channel kinetics of nicotinic acetylcholine receptors. Biophys J. 1988 Jan;53(1):1–10. doi: 10.1016/S0006-3495(88)83059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick J., McMillan J., Wolfson H., O'Brien J. C. Acetylcholine receptor metabolism in a nonfusing muscle cell line. J Biol Chem. 1977 Mar 25;252(6):2143–2153. [PubMed] [Google Scholar]
- Pennefather P., Quastel D. M. Fast desensitization of the nicotinic receptor at the mouse neuromuscular junction. Br J Pharmacol. 1982 Nov;77(3):395–404. doi: 10.1111/j.1476-5381.1982.tb09311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEPHENSON R. P. A modification of receptor theory. Br J Pharmacol Chemother. 1956 Dec;11(4):379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Schubert D., Harris A. J., Devine C. E., Heinemann S. Characterization of a unique muscle cell line. J Cell Biol. 1974 May;61(2):398–413. doi: 10.1083/jcb.61.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scubon-Mulieri B., Parsons R. L. Desensitization onset and recovery at the potassium-depolarized frog neuromuscular junction are voltage sensitive. J Gen Physiol. 1978 Mar;71(3):285–299. doi: 10.1085/jgp.71.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of a nicotinic acetylcholine receptor. Biophys J. 1984 Jan;45(1):175–185. doi: 10.1016/S0006-3495(84)84146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by high concentrations of agonist. J Physiol. 1987 Apr;385:325–359. doi: 10.1113/jphysiol.1987.sp016496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by low concentrations of agonist. J Physiol. 1986 Apr;373:129–162. doi: 10.1113/jphysiol.1986.sp016039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Agonists block currents through acetylcholine receptor channels. Biophys J. 1984 Aug;46(2):277–283. doi: 10.1016/S0006-3495(84)84022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Taylor P. Relationship between reversible antagonist occupancy and the functional capacity of the acetylcholine receptor. J Biol Chem. 1981 Jul 10;256(13):6692–6699. [PubMed] [Google Scholar]
- Sine S. M., Taylor P. The relationship between agonist occupation and the permeability response of the cholinergic receptor revealed by bound cobra alpha-toxin. J Biol Chem. 1980 Nov 10;255(21):10144–10156. [PubMed] [Google Scholar]
- Sine S., Taylor P. Functional consequences of agonist-mediated state transitions in the cholinergic receptor. Studies in cultured muscle cells. J Biol Chem. 1979 May 10;254(9):3315–3325. [PubMed] [Google Scholar]
- Trautmann A. A comparative study of the activation of the cholinergic receptor by various agonists. Proc R Soc Lond B Biol Sci. 1983 May 23;218(1211):241–251. doi: 10.1098/rspb.1983.0037. [DOI] [PubMed] [Google Scholar]
- Udgaonkar J. B., Hess G. P. Acetylcholine receptor: channel-opening kinetics evaluated by rapid chemical kinetic and single-channel current measurements. Biophys J. 1987 Nov;52(5):873–883. doi: 10.1016/S0006-3495(87)83281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udgaonkar J. B., Hess G. P. Chemical kinetic measurements of a mammalian acetylcholine receptor by a fast-reaction technique. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8758–8762. doi: 10.1073/pnas.84.24.8758. [DOI] [PMC free article] [PubMed] [Google Scholar]


