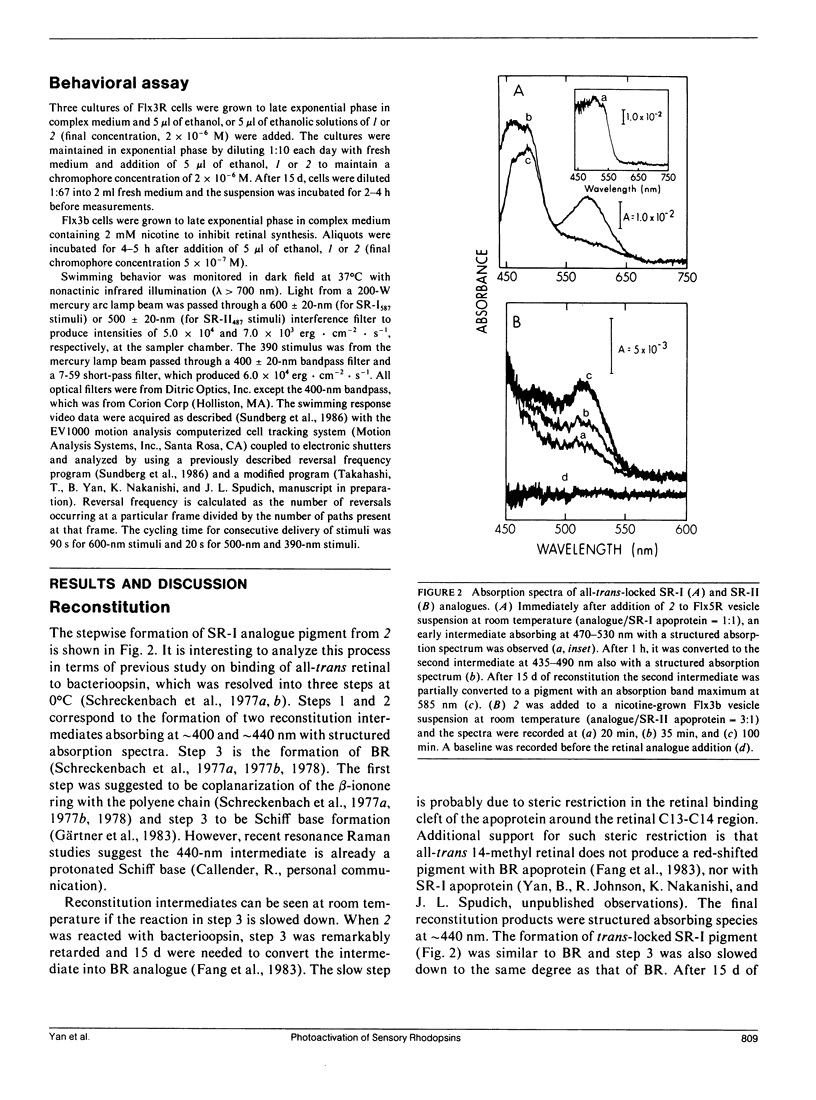
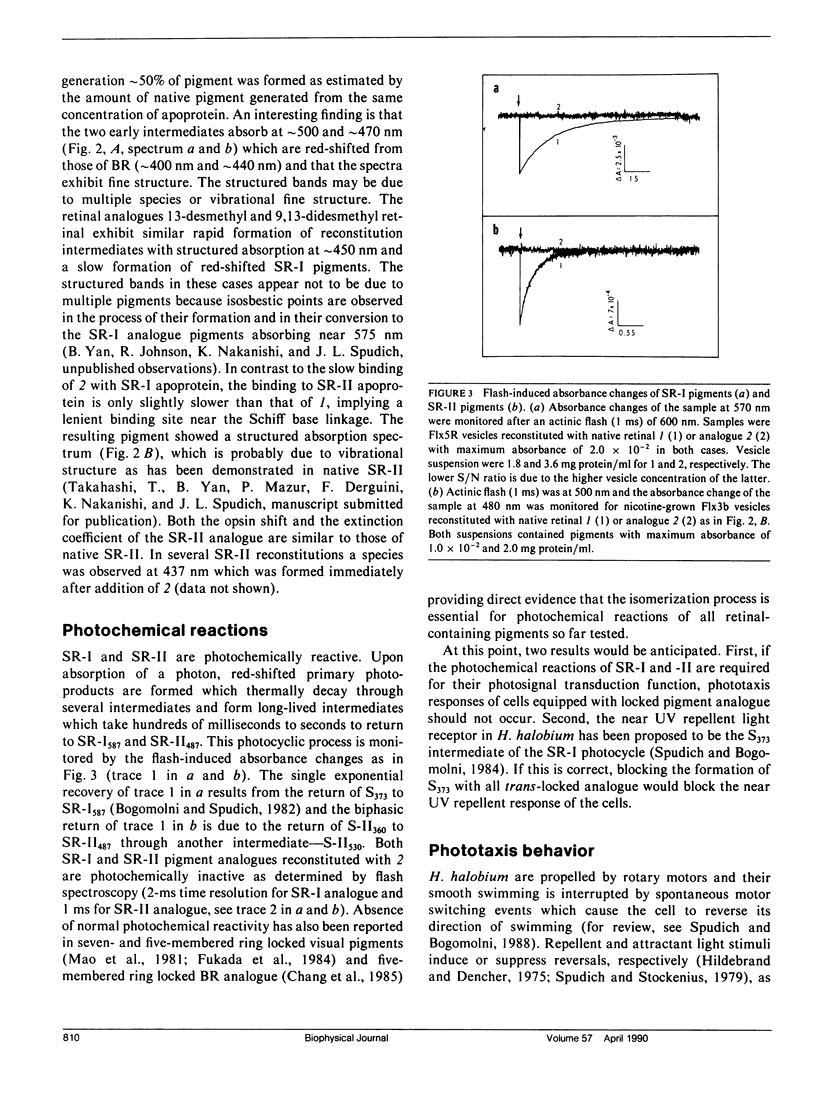
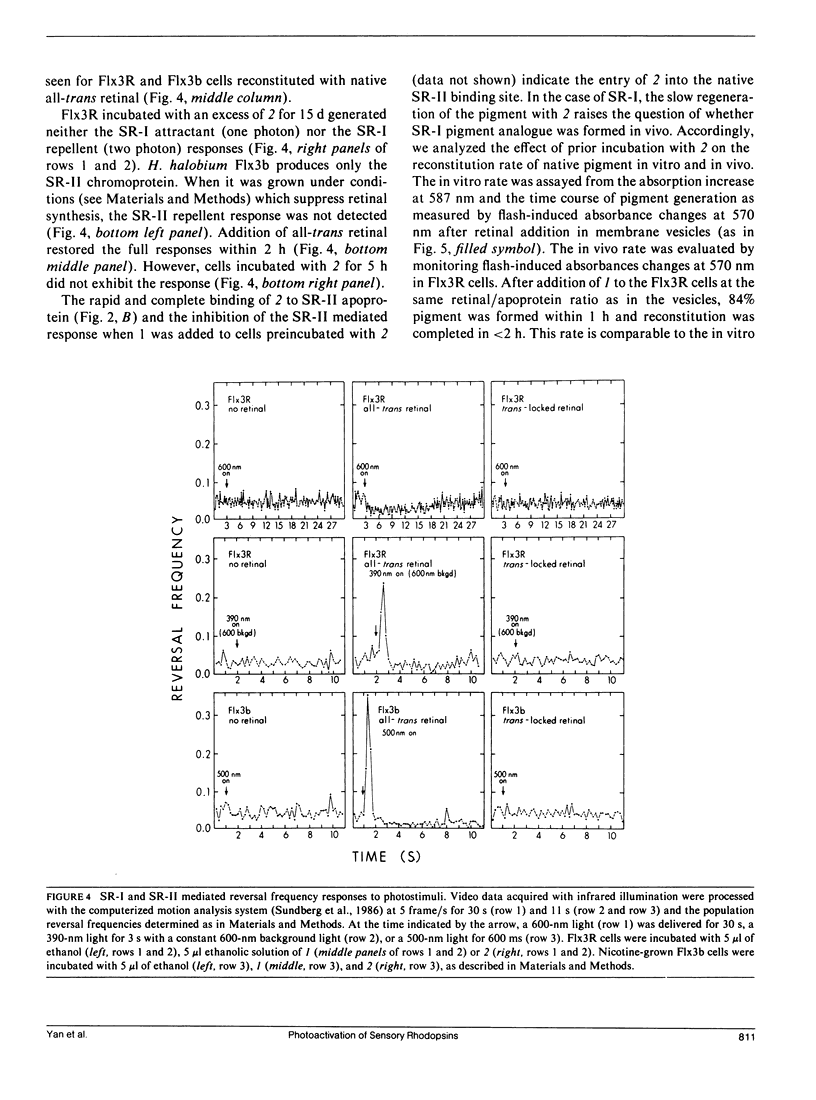
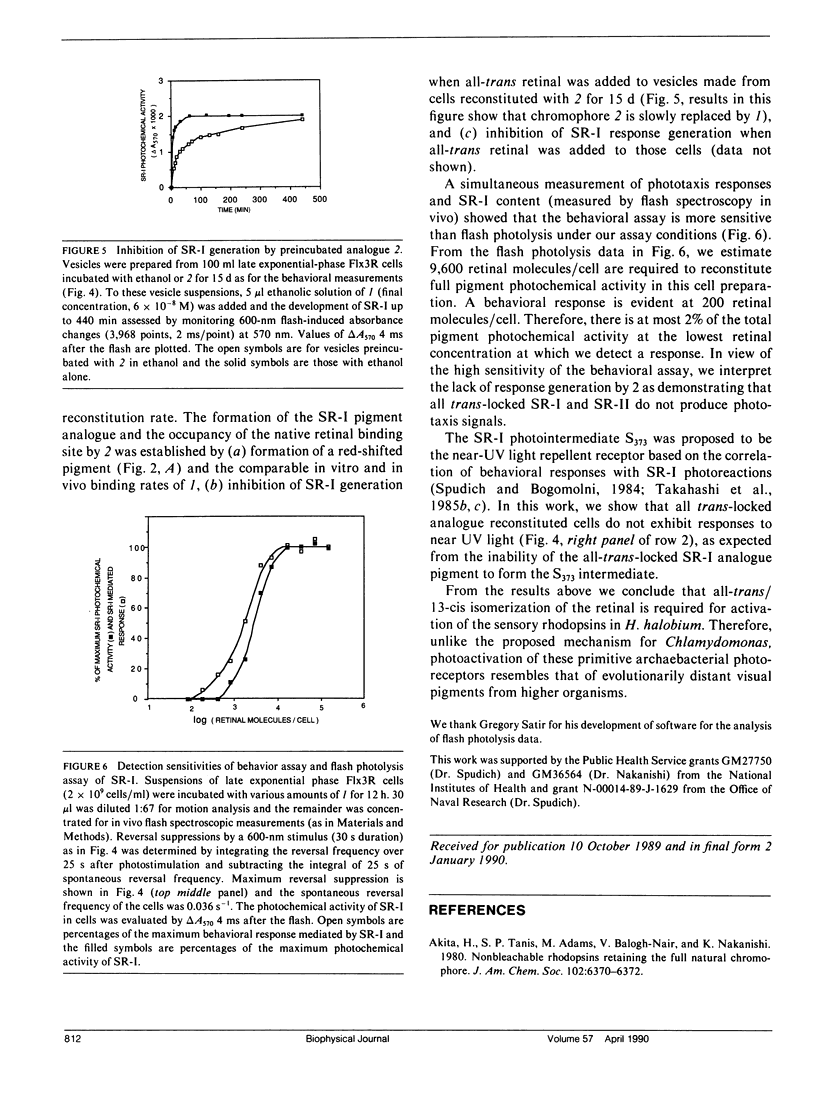
Abstract
An analogue of all-trans retinal in which all-trans/13-cis isomerization is blocked by a carbon bridge from C12 to C14 was incorporated into the apoproteins of sensory rhodopsin I (SR-I) and sensory rhodopsin II (SR-II, also called phoborhodopsin) in retinal-deficient Halobacterium halobium membranes. The "all-trans-locked" retinal analogue forms SR-I and SR-II analogue pigments with similar absorption spectra as the native pigments. Blocking isomerization prevents the formation of the long-lived intermediate of the SR-I photocycle (S373) and those of the SR-II photocycle (S-II360 and S-II530). A computerized cell tracking and motion analysis system capable of detecting 2% of native pigment activity was used for assessing motility behavior. Introduction of the locked analogue into SR-I or SR-II apoprotein in vivo did not restore phototactic responses through any of the three known photosensory systems (SR-I attractant, SR-I repellent, or SR-II repellent). We conclude that unlike the phototaxis receptor of Chlamydomonas reinhardtii, which has been reported to mediate physiological responses without specific double-bond isomerization of its retinal chromophore (Foster et al., 1989), all-trans/13-cis isomerization is essential for SR-I and SR-II phototaxis signaling.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagley K. A., Balogh-Nair V., Croteau A. A., Dollinger G., Ebrey T. G., Eisenstein L., Hong M. K., Nakanishi K., Vittitow J. Fourier-transform infrared difference spectroscopy of rhodopsin and its photoproducts at low temperature. Biochemistry. 1985 Oct 22;24(22):6055–6071. doi: 10.1021/bi00343a006. [DOI] [PubMed] [Google Scholar]
- Birge R. R. Photophysics of light transduction in rhodopsin and bacteriorhodopsin. Annu Rev Biophys Bioeng. 1981;10:315–354. doi: 10.1146/annurev.bb.10.060181.001531. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A., Spudich J. L. Identification of a third rhodopsin-like pigment in phototactic Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6250–6254. doi: 10.1073/pnas.79.20.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M., Mathies R. Resonance Raman spectra of bacteriorhodopsin's primary photoproduct: evidence for a distorted 13-cis retinal chromophore. Proc Natl Acad Sci U S A. 1982 Jan;79(2):403–407. doi: 10.1073/pnas.79.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabre M. Trigger and amplification mechanisms in visual phototransduction. Annu Rev Biophys Biophys Chem. 1985;14:331–360. doi: 10.1146/annurev.bb.14.060185.001555. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Govindjee R., Ebrey T., Bagley K. A., Dollinger G., Eisenstein L., Marque J., Roder H., Vittitow J., Fang J. M. Trans/13-cis isomerization is essential for both the photocycle and proton pumping of bacteriorhodopsin. Biophys J. 1985 Apr;47(4):509–512. doi: 10.1016/S0006-3495(85)83944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch R., Katz S., Nakanishi K., Gawinowicz M. A., Balogh-Nair V. Incorporation of 11,12-dihydroretinal into the retinae of vitamin a deprived rats. Photochem Photobiol. 1981 Jan;33(1):91–95. doi: 10.1111/j.1751-1097.1981.tb04302.x. [DOI] [PubMed] [Google Scholar]
- Eyring G., Curry B., Broek A., Lugtenburg J., Mathies R. Assignment and interpretation of hydrogen out-of-plane vibrations in the resonance Raman spectra of rhodopsin and bathorhodopsin. Biochemistry. 1982 Jan 19;21(2):384–393. doi: 10.1021/bi00531a028. [DOI] [PubMed] [Google Scholar]
- Eyring G., Curry B., Mathies R., Fransen R., Palings I., Lugtenburg J. Interpretation of the resonance Raman spectrum of bathorhodopsin based on visual pigment analogues. Biochemistry. 1980 May 27;19(11):2410–2418. doi: 10.1021/bi00552a020. [DOI] [PubMed] [Google Scholar]
- Fodor S. P., Bogomolni R. A., Mathies R. A. Structure of the retinal chromophore in the hRL intermediate of halorhodopsin from resonance raman spectroscopy. Biochemistry. 1987 Oct 20;26(21):6775–6778. doi: 10.1021/bi00395a029. [DOI] [PubMed] [Google Scholar]
- Foster K. W., Saranak J., Derguini F., Zarrilli G. R., Johnson R., Okabe M., Nakanishi K. Activation of Chlamydomonas rhodopsin in vivo does not require isomerization of retinal. Biochemistry. 1989 Jan 24;28(2):819–824. doi: 10.1021/bi00428a061. [DOI] [PubMed] [Google Scholar]
- Foster K. W., Saranak J., Patel N., Zarilli G., Okabe M., Kline T., Nakanishi K. A rhodopsin is the functional photoreceptor for phototaxis in the unicellular eukaryote Chlamydomonas. Nature. 1984 Oct 25;311(5988):756–759. doi: 10.1038/311756a0. [DOI] [PubMed] [Google Scholar]
- Fukada Y., Shichida Y., Yoshizawa T., Ito M., Kodama A., Tsukida K. Studies on structure and function of rhodopsin by use of cyclopentatrienylidene 11-cis-locked-rhodopsin. Biochemistry. 1984 Nov 20;23(24):5826–5832. doi: 10.1021/bi00319a023. [DOI] [PubMed] [Google Scholar]
- Gawinowicz M. A., Balogh-Nair V., Sabol J. S., Nakanishi K. A nonbleachable rhodopsin analogue formed from 11, 12-dihydroretinal. J Am Chem Soc. 1977 Nov 9;99(23):7720–7721. doi: 10.1021/ja00465a059. [DOI] [PubMed] [Google Scholar]
- Hayward G., Carlsen W., Siegman A., Stryer L. Retinal chromophore of rhodopsin photoisomerizes within picoseconds. Science. 1981 Feb 27;211(4485):942–944. doi: 10.1126/science.7466366. [DOI] [PubMed] [Google Scholar]
- Hildebrand E., Dencher N. Two photosystems controlling behavioural responses of Halobacterium halobium. Nature. 1975 Sep 4;257(5521):46–48. doi: 10.1038/257046a0. [DOI] [PubMed] [Google Scholar]
- Howes C. D., Batra P. P. Accumulation of lycopene and inhibition of cyclic carotenoids in Mycobacterium in the presence of nicotine. Biochim Biophys Acta. 1970 Oct 27;222(1):174–179. doi: 10.1016/0304-4165(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Hubbard R., Kropf A. THE ACTION OF LIGHT ON RHODOPSIN. Proc Natl Acad Sci U S A. 1958 Feb;44(2):130–139. doi: 10.1073/pnas.44.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K. Halorhodopsin: a light-driven chloride ion pump. Annu Rev Biophys Biophys Chem. 1986;15:11–28. doi: 10.1146/annurev.bb.15.060186.000303. [DOI] [PubMed] [Google Scholar]
- Manor D., Hasselbacher C. A., Spudich J. L. Membrane potential modulates photocycling rates of bacterial rhodopsins. Biochemistry. 1988 Aug 9;27(16):5843–5848. doi: 10.1021/bi00416a004. [DOI] [PubMed] [Google Scholar]
- Mao B., Tsuda M., Ebrey T. G., Akita H., Balogh-Nair V., Nakanishi K. Flash photolysis and low temperature photochemistry of bovine rhodopsin with a fixed 11-ene. Biophys J. 1981 Aug;35(2):543–546. doi: 10.1016/S0006-3495(81)84809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettei M. J., Yudd A. P., Nakanishi K., Henselman R., Stoeckenius W. Identification of retinal isomers isolated from bacteriorhodopsin. Biochemistry. 1977 May 3;16(9):1955–1959. doi: 10.1021/bi00628a031. [DOI] [PubMed] [Google Scholar]
- Schreckenbach T., Oesterhelt D. Photochemical and chemical studies on the chromophore of bacteriorhodopsin. Fed Proc. 1977 May;36(6):1810–1814. [PubMed] [Google Scholar]
- Schreckenbach T., Walckhoff B., Oesterhelt D. Specificity of the retinal binding site of bacteriorhodopsin: chemical and stereochemical requirements for the binding of retinol and retinal. Biochemistry. 1978 Dec 12;17(25):5353–5359. doi: 10.1021/bi00618a005. [DOI] [PubMed] [Google Scholar]
- Schreckenbach T., Walckhoff B., Oesterhelt D. Studies on the retinal-protein interaction in bacteriorhodopsin. Eur J Biochem. 1977 Jun 15;76(2):499–511. doi: 10.1111/j.1432-1033.1977.tb11620.x. [DOI] [PubMed] [Google Scholar]
- Spudich E. N., Sundberg S. A., Manor D., Spudich J. L. Properties of a second sensory receptor protein in Halobacterium halobium phototaxis. Proteins. 1986 Nov;1(3):239–246. doi: 10.1002/prot.340010306. [DOI] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Mechanism of colour discrimination by a bacterial sensory rhodopsin. Nature. 1984 Dec 6;312(5994):509–513. doi: 10.1038/312509a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Sensory rhodopsins of halobacteria. Annu Rev Biophys Biophys Chem. 1988;17:193–215. doi: 10.1146/annurev.bb.17.060188.001205. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Wolff E. K., Hess B. A rapid population method for action spectra applied to Halobacterium halobium. J Bacteriol. 1988 Jun;170(6):2790–2795. doi: 10.1128/jb.170.6.2790-2795.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg S. A., Alam M., Spudich J. L. Excitation signal processing times in Halobacterium halobium phototaxis. Biophys J. 1986 Nov;50(5):895–900. doi: 10.1016/S0006-3495(86)83530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Mochizuki Y., Kamo N., Kobatake Y. Evidence that the long-lifetime photointermediate of s-rhodopsin is a receptor for negative phototaxis in Halobacterium halobium. Biochem Biophys Res Commun. 1985 Feb 28;127(1):99–105. doi: 10.1016/s0006-291x(85)80131-5. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Watanabe M., Kamo N., Kobatake Y. Negative phototaxis from blue light and the role of third rhodopsinlike pigment in halobacterium cutirubrum. Biophys J. 1985 Aug;48(2):235–240. doi: 10.1016/S0006-3495(85)83776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Nelson B., Chang C. H., Govindjee R., Ebrey T. G. Characterization of the chromophore of the third rhodopsin-like pigment of Halobacterium halobium and its photoproduct. Biophys J. 1985 May;47(5):721–724. doi: 10.1016/S0006-3495(85)83969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G. The molecular basis of visual excitation. Nature. 1968 Aug 24;219(5156):800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]


